Main Body
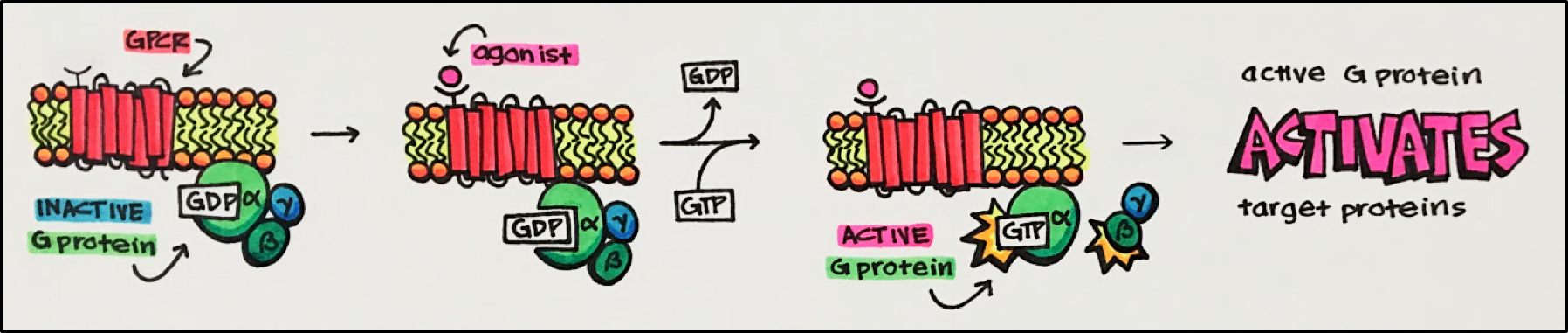
G-Protein-Coupled Receptors [GPCRs] • largest family of transmembrane proteins in the human genome with more than 800 unique GPCRs. These receptors are coupled to intracellular GTP-binding proteins (G-proteins). Once activated, G-proteins trigger the production of a variety of second messengers (e.g. cyclic AMP [cAMP], inositol triphosphate [IP3], diacylglycerol [DAG], etc.) helping to regulate a number of body functions ranging from sensation to growth to hormone release.
GPCR Structure • each GPCR is composed of 7 transmembrane helices connected by extracellular and intracellular loops
G-proteins • G proteins are made of aßg-trimers. In a resting state guanosine diphosphate (GDP) is bound to this trimer. Upon receptor activation by an agonist the G protein is attracted to the receptor. This leads to guanosine triphosphate (GTP) displacing GDP binding on the alpha subunit to activate the G protein by dissociating the a subunit from the ßg dimer.
- Ga Subunit • different subtypes. Inhibits or stimulates adenylyl cyclase, activates phospholipase C, inhibits voltage-gated calcium channels, activates GPCR kinases, activates mitogen-activated protein kinase cascade.
- ßg Subunit dimer • activates potassium channels, and some protein kinases.
Subtypes of alpha subunits of G-proteins
| G-protein Subtype | Receptors with which the
G-protein Subtype Associates |
Main Effectors |
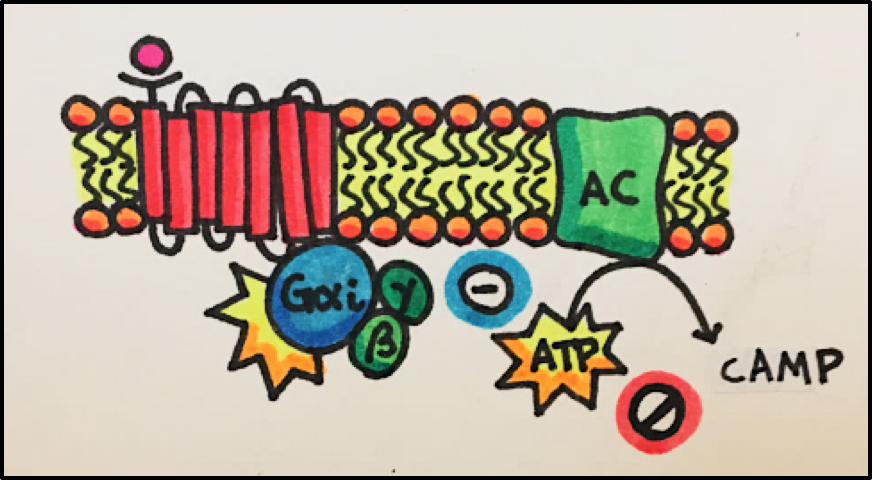
| Gai | cannabinoid, catecholamine, histamine, opioid, serotonin, and other receptors | inhibits adenylyl cyclase to decrease cAMP formation |
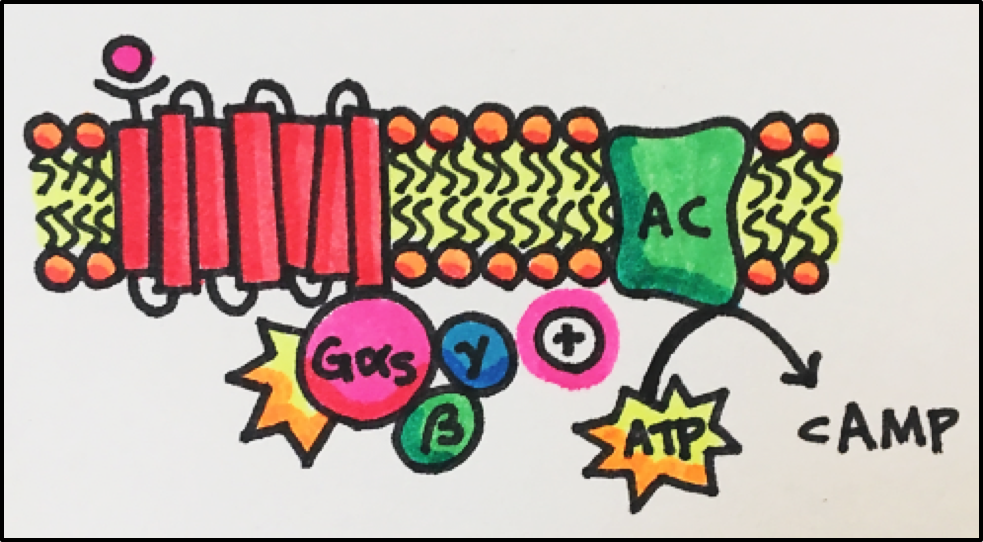
| Gas | catecholamine, histamine, serotonin and other receptors | stimulates adenylyl cyclase to increase cAMP formation |
| Gao | cannabinoid, catecholamine, histamine, opioid, serotonin, and other receptors | Gao has limited effects that are similar to GaI, rather ßg elicits responses |
| Gaq | amine, peptide and prostanoid receptors | activates PLC (phospholipase C) increasing the production of a second messengers IP3 (inositol triphosphate) and DAG (diacylglycerol) |
| G12/13 | angiotensin II, muscarinic [M1 and M3], purinergic, serotonin, thromboxane, thrombin and more | activates RhoGEF, which goes on to activate Rho kinase |
Main G-protein Targets
- Adenylyl Cyclase [AC] • enzyme catalyzing cAMP formation
- Ion Channels • mainly calcium and potassium channels
- Mitogen-activated Protein Kinases [MAP Kinases] • system that controls a number of cell functions including cell division
- Phospholipase C [PLC] • enzyme that catalyzes breakdown of certain membrane lipid components to form inositol trisphosphate (IP3) that releases calcium from intracellular stores and diacylglycerol (DAG) that activates protein kinase C
- RhoA/Rho Kinase • system that controls the activity of a number of signaling pathways that in turn control cell growth and proliferation, smooth muscle contraction and more.
GPCR Pathways
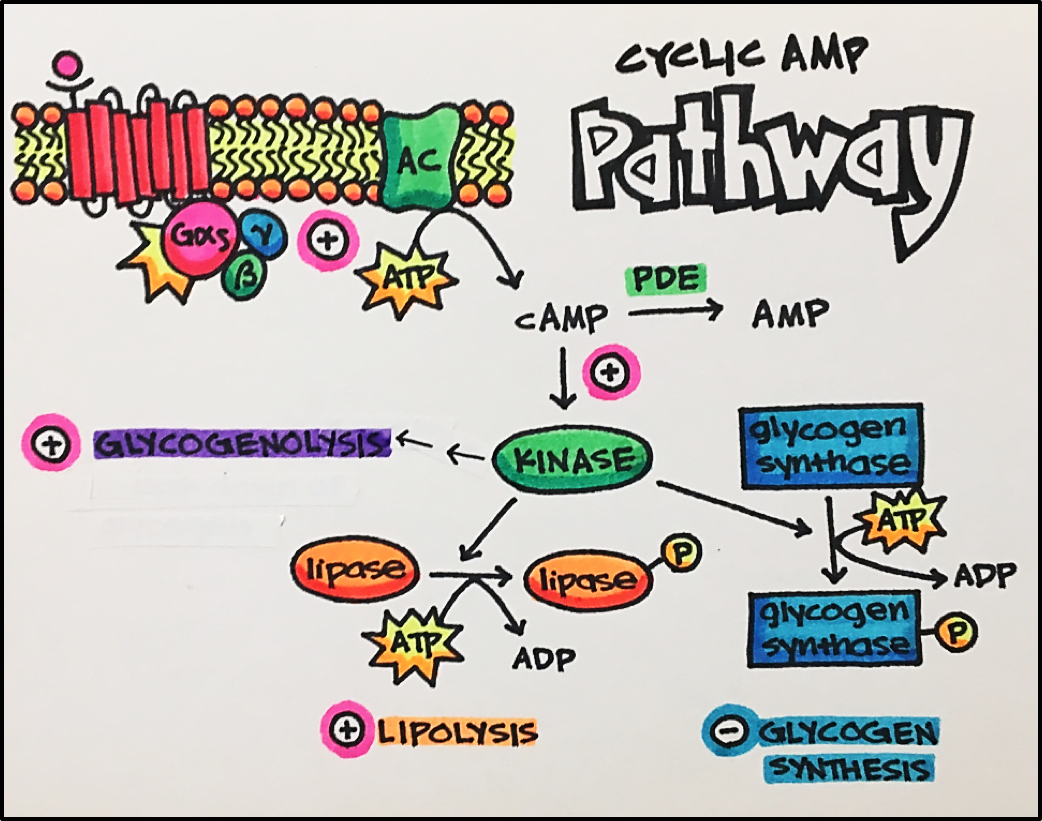
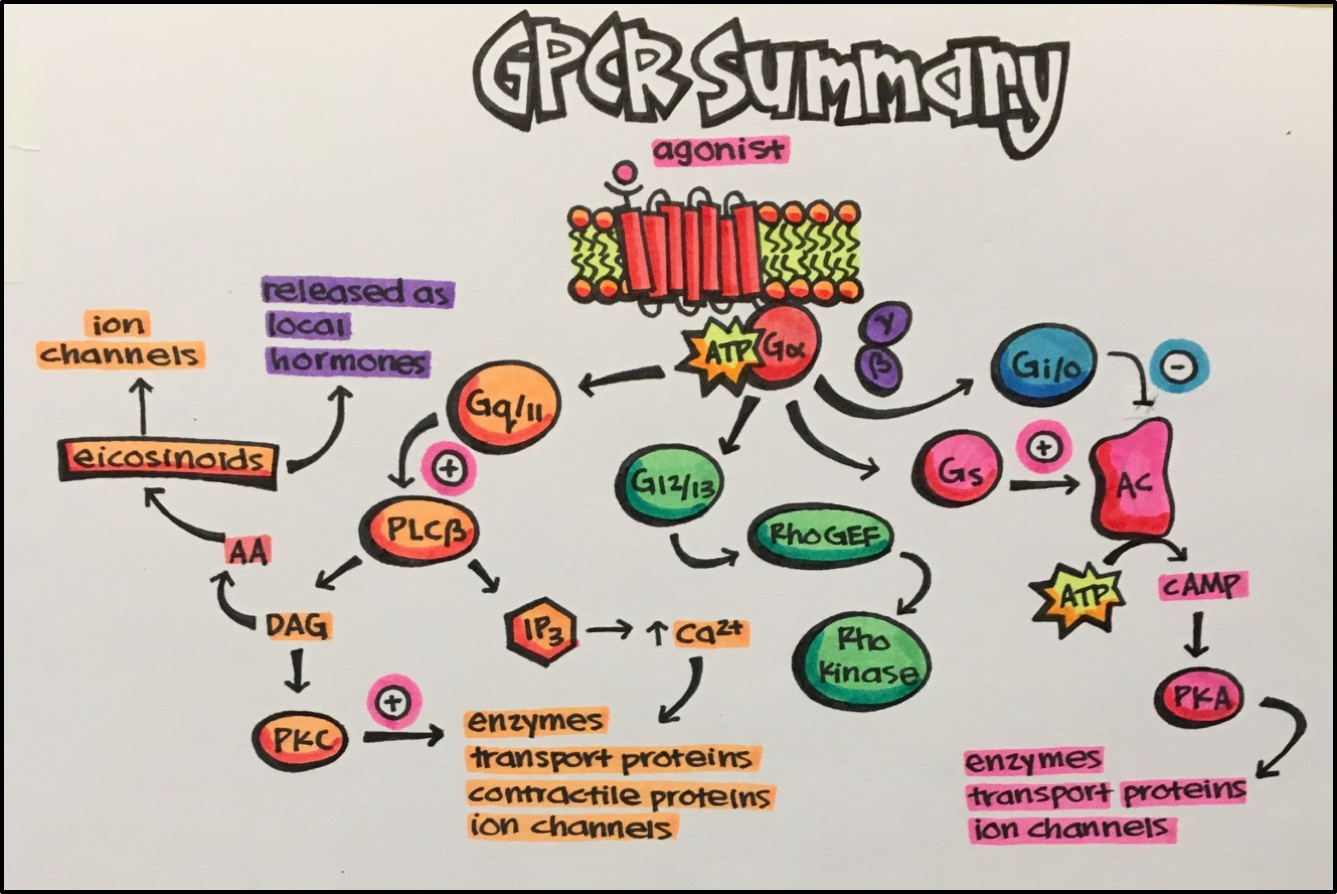
Cyclic AMP Pathway • An agonist stimulates the GPCR, which them activates the G-protein (Gas or Gai). Gas will go on to stimulate its target protein, adenylyl cyclase [AC], which catalyzes the conversion of ATP to cAMP. Gai inhibits adenylyl cyclase, which decreases cAMP (cyclic AMP) levels.
Cyclic AMP activates protein kinases, one important target is protein kinase A (PKA). Active protein kinases have a variety of effects including lipolysis (break down of lipid molecules), reduction in glycogen synthesis (one way our body stores glucose), and increased glycogenolysis (break down of glycogen to increase blood glucose levels). Protein kinases act to regulate the function of many different cellular proteins by controlling protein phosphorylation to regulate homeostasis.
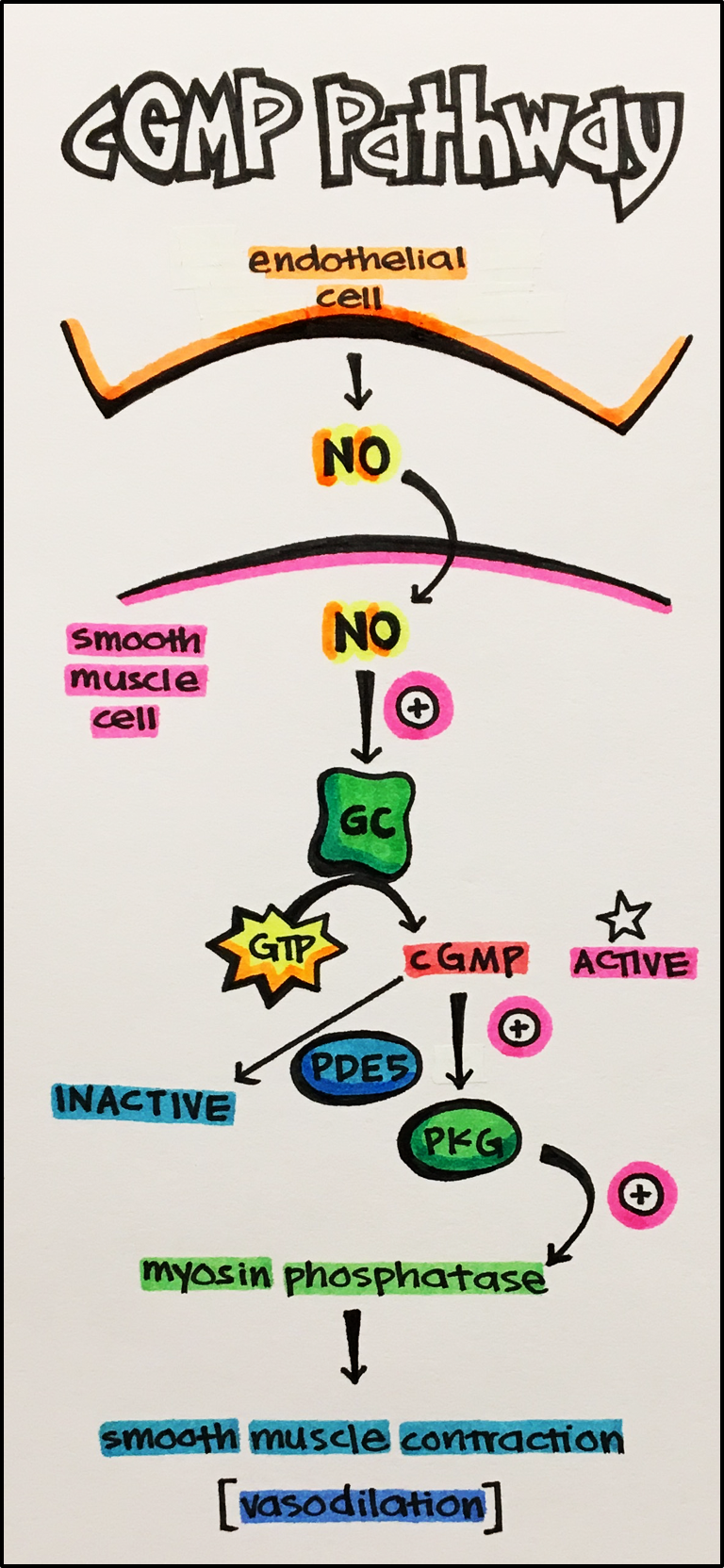
Cyclic GMP [cGMP] Pathway • Nitric oxide [NO] is a small transmitter that diffuses from one cell to another to mediate chemical communication. It is formed from the amino acid L-arginine through the action of the enzyme NO synthase. NO activates guanylate cyclase (GC) that results in the formation of cGMP from GTP. The main function of cGMP is dilation of blood vessels through activation of protein kinases. For example, NO is produced by endothelial cells outlining the interior of blood vessels, then diffuses to the smooth muscle layer of blood vessels to cause vasodilation. cGMP is inactivated when hydrolyzed by a group of phosphodiesterase enzymes (PDEs) that are differentially expressed in various tissues.
This pathway provides an important drug target for the treatment of angina; chest pain caused by reduced blood supply to the heart due vasoconstriction of the coronary arteries. Therefore, organonitrates (e.g. nitroglycerin) that release NO are effective in the treatment of angina pain. Inhibitors of cGMP hydrolysis by phosphodiesterase inhibitors (PDEIs) represent another important class of drugs. For instance, slidenafil (Trade name: Viagra) selectively inhibits PDEs that are preferentially expressed in blood vessels in the penis. This results in vasodilation to cause erection. Such drugs are therefore effective in the treatment of erectile disorders.
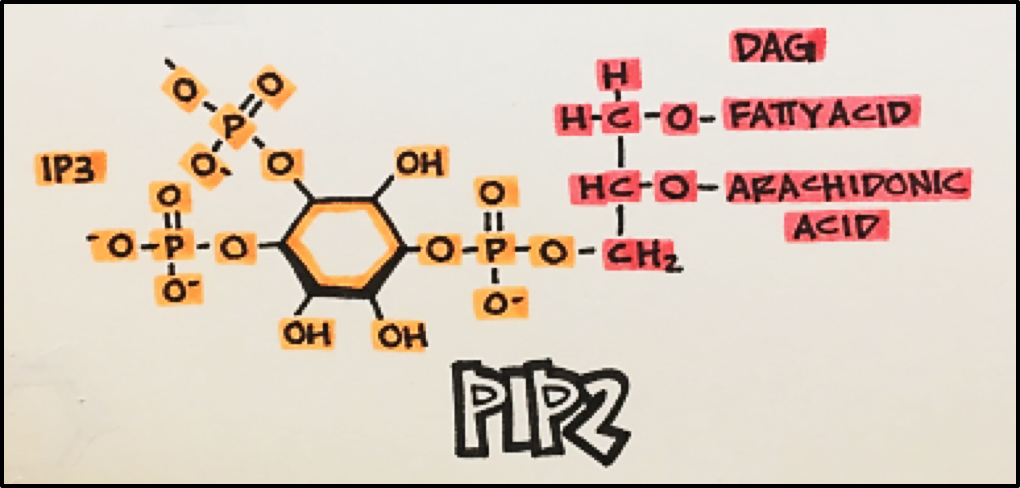
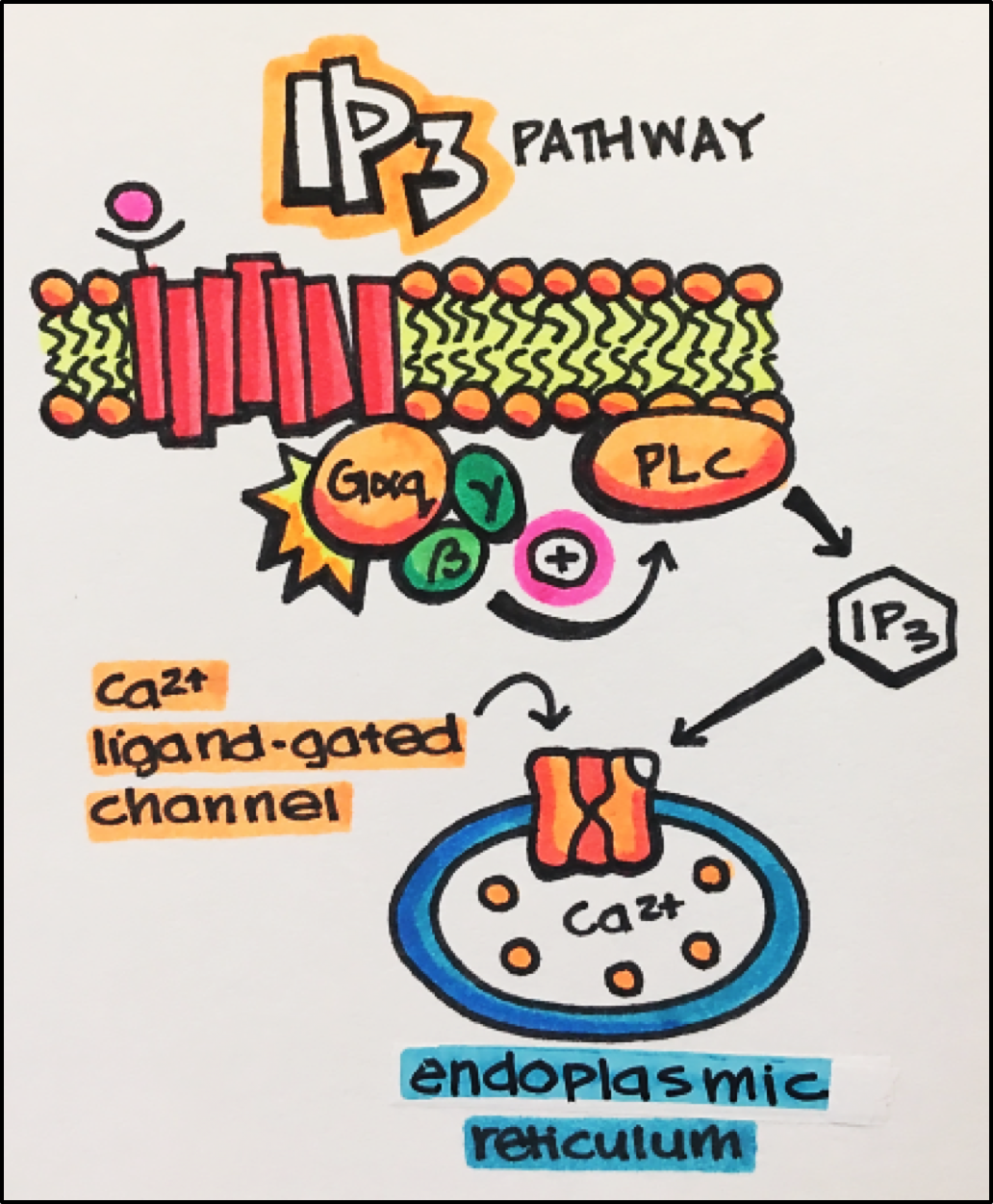
PLC Pathway • This pathway involves the G-protein Gaq. When activated, Gaq goes on to activate phospholipase C, which catalyzes the formation of two intracellular messengers, IP3 and DAG, from PIP2 (phosphatidylinositol 4,5-bisphosphonate).
IP3 (inositol triphosphate) increases free cytosolic Ca2+ by releasing Ca2+ from intracellular compartments. IP3 is an endogenous ligand for calcium ligand-gated channels imbedded in the endoplasmic and sarcoplasmic reticulum in smooth and skeletal muscle, respectively. Upon binding, IP3 opens the channel to release free calcium into the cytosol. Ca2+ controls a number of events including: muscle contraction, secretion, enzyme activation, and membrane hyperpolarization.
DAG (diacylglycerol) activates protein kinase C, which controls a number of cellular functions by phosphorylating a variety of target proteins.
Rho Kinase
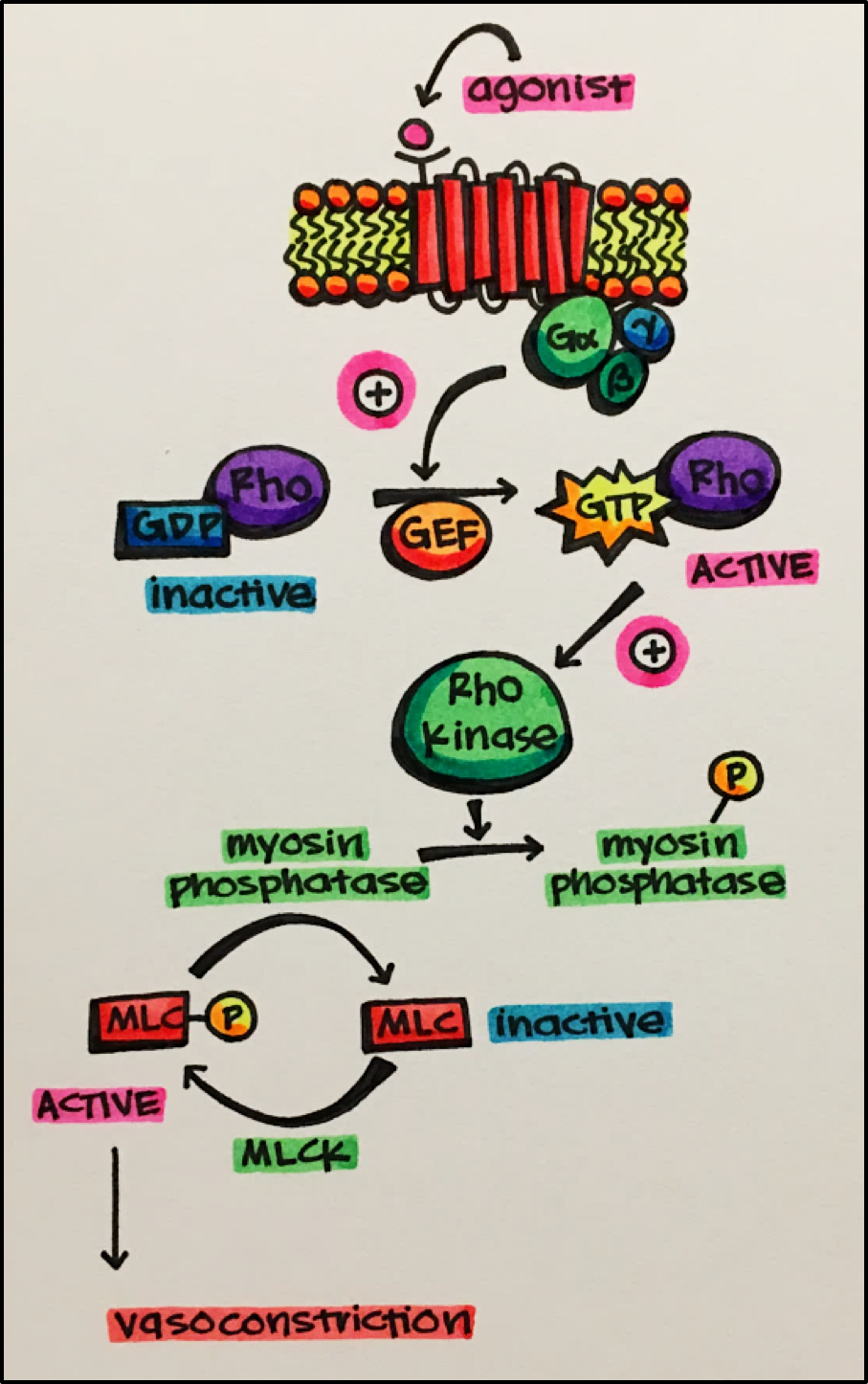
Rho Kinase • this kinase is activated by GPCRs that are coupled to G12/G13 G-proteins. G12/G13 G-proteins regulate cell processes using guanine nucleotide exchange factors (GEFs).
- The free G-protein alpha subunit interacts with a guanosine nucleotide exchange factor that facilitates the GDP-GTP exchange at another GTPase known as Rho.
- Rho-GDP (resting form) is activated when the GDP-GTP exchange occurs. The activated GTPase Rho goes on to activated Rho kinase.
- Rho kinase phosphorylates a number of substrate proteins. This phosphorylation controls a number of cellular functions such as:
- o Smooth muscle contraction and proliferation
- o Angiogenesis and synaptic remodeling
- o Hypothesized to be important in the pathogenesis of pulmonary hypotension
- Rho kinase inhibitors are an up-and-coming area of drug development with a wide range of clinical indications
- Example • Fasudil • a selective Rho-Kinase inhibitor indicated for the management of cerebrovascular disorders such as vasospasm post-surgery for subarachnoid hemorrhage. It is currently under investigation for angina, acute cerebral thrombosis and pulmonary hypertension.
Phospholipase A2
GPCRs also have a role in controlling phospholipase A2 and the synthesis of arachidonic acid (further synthesized into a number inflammatory mediators and modulators) and eicosanoids.
Eicosanoids • Eicosanoids are considered “local hormones” as their action takes place on nearby cells (paracrine hormones). Eicosanoids are involved with intracellular signaling by stimulating GPCRs coupled to G-protein subtypes Gs, Gq and G12/G13. They play a role in cardiovascular disease, triglyceride [TAG] levels, blood pressure and arthritis.
- Examples of Eicosanoids • leukotrienes, prostaglandins and thromboxanes
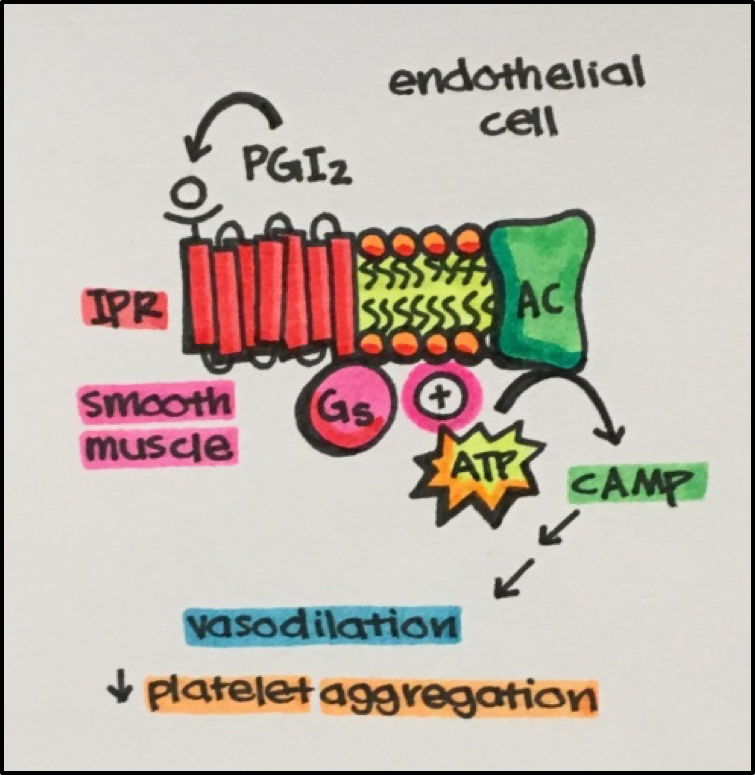
- Prostaglandins stimulate GPCRs coupled to Gs resulting in vasodilation and decreased platelet aggregation.
See Right • Prostaglandin I2 (PGI2) binds to the prostaglandin I2 receptor [IPR], which is a GPCR, eventually leading to vasodilation of smooth muscle as well as decreased platelet aggregation (preventing clot, and possible thrombosis, formation).
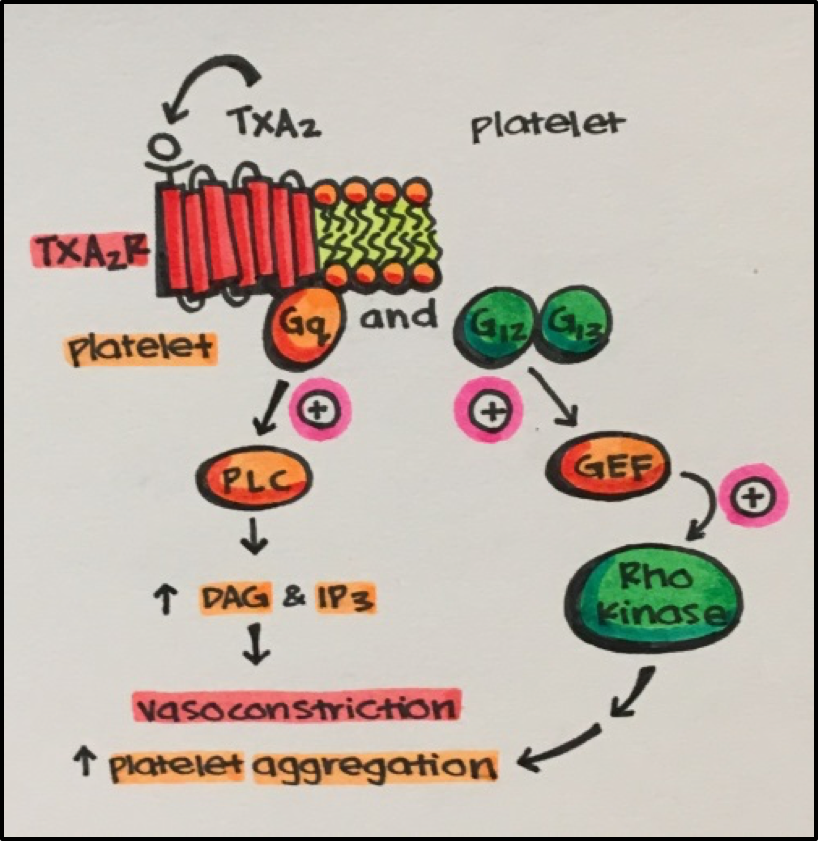
- Thromboxanes stimulate GPCRs coupled to Gq and G12/G13 resulting in vasoconstriction, increased platelet aggregation, which can lead to thrombosis (blood clot).
See Right • Thromboxane A2 [TXA2] bind to the thromboxane A2 receptor [TXA2R] resulting in a number of events that can potentially lead to interruption of blood flow (thrombosis) (e.g., vasoconstriction and platelet aggregation). Thrombosis can lead to more serious events such as stroke.
As you can probably imagine, it is essential that an appropriate balance of prostaglandins and thrombonxanes is maintained to avoid pathogenesis including cardiovascular disease or stroke.
GPCR SUMMARY
In summary, GPCRs are transmembrane receptors that allow for extracellular signals to be communicated (by signal transduction) to intracellular effectors that eventually lead to a particular cellular response. As you can see above, each G-protein subtype initiates a particular signaling pathway. Each of these pathways leads to a variety of events such as: cell growth, proliferation, and differentiation, vasoconstriction or vasodilation, platelet aggregation, and muscle contraction to name a few. Hopefully you can see why GPCRs are such an important drug target.