Main Body
13. Enzyme-Linked Receptors
Enzyme-Linked Receptors • have intrinsic enzymatic activity or are associated with an enzyme (usually a kinase) • play a role in apoptosis, cell differentiation, cell division, cell growth, immune response, inflammation, and tissue repair.
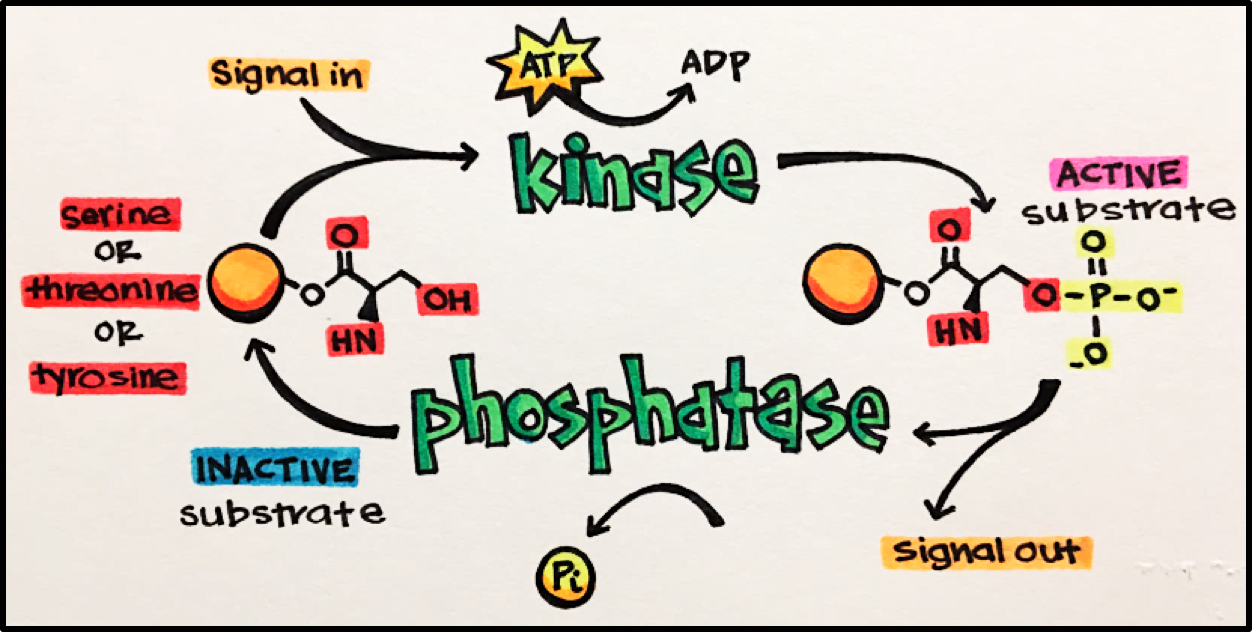
Kinases (Protein Kinases [PKs]) • enzymes that catalyze the phosphorylation of target molecules to cause their activation. In other words, they add a phosphate group to a molecule/protein/another kinase.
- Protein Kinase Inhibitors (PKIs): stop kinase activity, important class of molecules used for cancer treatment
Phosphatases • enzymes that catalyze the dephosphorylation of target molecules. This dephosphorylation usually inactivates the target molecule (effector). Phosphatases act in opposition to kinases.
It is the balanced activity of kinases and phosphatases that results in effective, and fast, signaling events to allow for the desired biological effect and enable our bodies to maintain homeostasis.
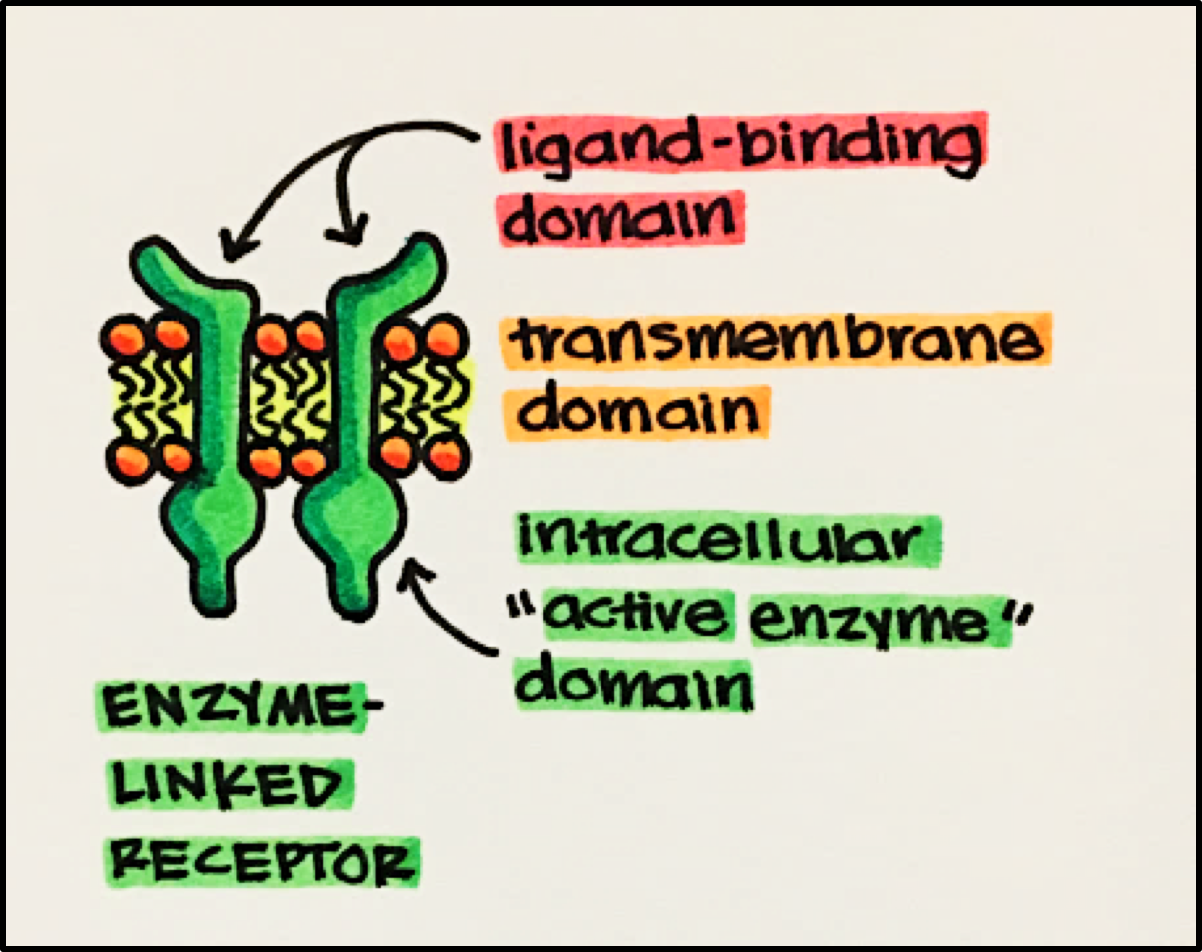
Enzyme Linked Receptor Structure • composed of three key domains
- Ligand-Binding Domain • often has a large EXTRACELLULAR ligand-binding domain allowing for easy access and activation to the receptor.
- Transmembrane Domain • composed of a series of hydrophobic amino acids (reminder: inner membrane is lipophilic/hydrophobic) that tethers the receptor to the cell membrane.
- Intracellular “Active Enzyme” Domain • either intrinsic to the receptor or tightly bound to the transmembrane domain. The majority of the “active enzyme” domains are kinases that phosphorylate the amino acids serine, threonine and tyrosine of proteins.
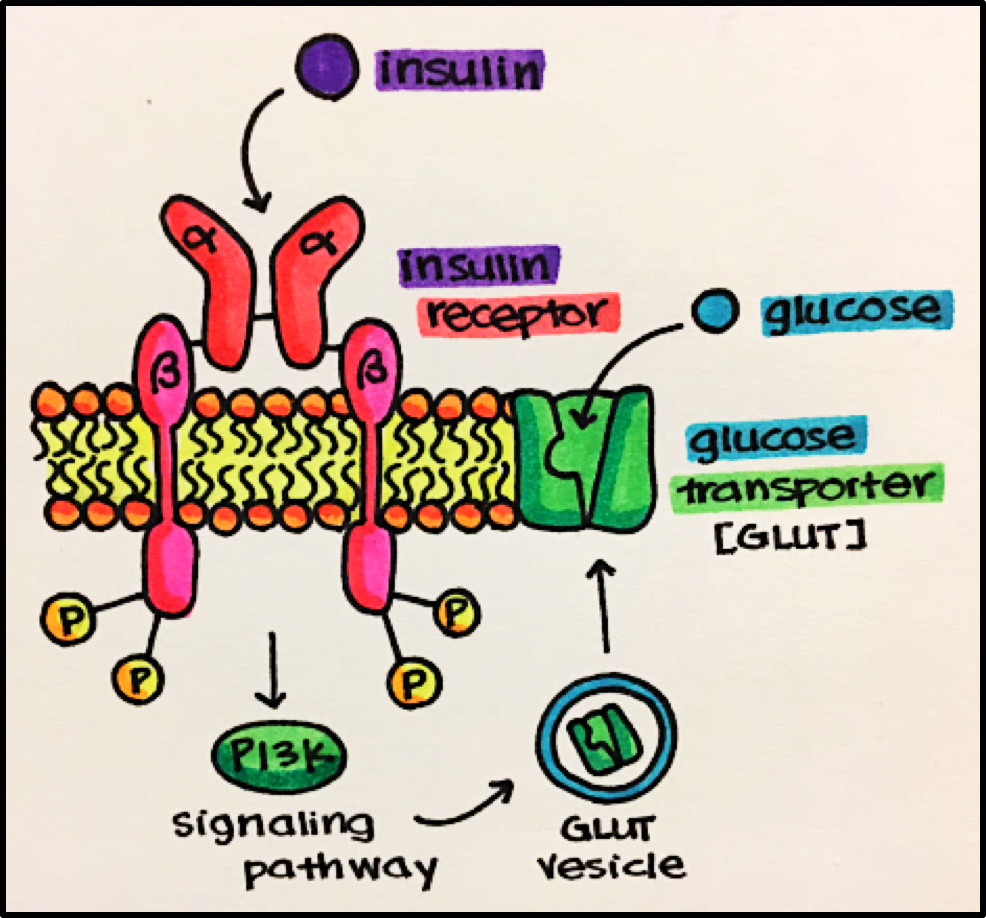
Example • Insulin Receptor • ligand is insulin, which stimulates carbohydrate (glucose) utilization and protein synthesis.
Ligand-binding domain = alpha domain
Transmembrane domain = runs through the membrane anchoring it to the membrane
“Active enzyme” domain = tyrosine kinase domain (intracellular component) activated by phosphorylation
Once activated, the insulin receptor leads to a cascade of events eventually resulting in expression of glucose transporters (GLUTs) on the surface of a cell to allow it to bring in glucose for energy utilization.
Signal Transduction by Enzyme-Linked Receptors
- Ligand binding leads to dimerization of two neighboring receptors.
- Neighboring dimerized receptors auto phosphorylate one another
- SH2-domain proteins bind to the phosphorylated receptors and are then phosphorylated enabling the continuation of the signal eventually leading to gene transcription.
- SH2-domain [Scr homology 2] • protein domain composed of about 100 amino acid residues. SH2-domains most commonly play a role in the signal transduction by receptor tyrosine kinase pathways as you will see later on in this section. They interact with specific target molecules (peptides) with a phosphorylated tyrosine residue.
3 Main Types of Enzyme-Linked Receptors •
- Receptor Tyrosine Kinases [RTK] • make up the majority of enzyme-linked receptors. Signal transduction through RTK results in specific phosphorylation of tyrosine residues on target proteins and subsequent increase in gene transcription and regulation of cell growth, differentiation and survival.
- Serine/Threonine Kinases • specifically phosphorylate the hydroxyl side chains of serine or threonine amino acid residues. These kinases may have a role in cell proliferation, differentiation, apoptosis and even embryonic development.
- Tyrosine Kinase-Associated Receptors [“mixed”] • do not have a tyrosine kinase domain, rather act through cytoplasmic tyrosine kinases.
Receptor Tyrosine Kinases [RTK] • This class of receptors are also considered enzymes that have intrinsic enzymatic activity. When RTK agonists bind to these receptors, their intrinsic enzymatic activity is stimulated. RTKs bind growth factors to signal processes that result in the regulation of cell growth, differentiation and survival through gene transcription. Subclasses include:
- Epidermal Growth Factor Receptors [EGFR, ErbB1, HER1] • This RTK subclass is activated by epidermal growth factor resulting in cell division that leads to cell growth, proliferation and differentiation. These receptors are found in abnormally high levels on the surface of a number of types of cancer cells allowing these cells to multiply excessively.
- Nerve Growth Factor Receptors [NGF] • This RTK subclass is activated by nerve growth factors, more specifically neurotropins; a family of proteins involved with development, survival and function of neurons.
- Insulin Receptors • This RTK subclass is activated by insulin resulting in expression of glucose transporters [GLUT] and allow cells to accumulate glucose from the blood.
- Toll Like Receptors [TLRs]• This RTK subclass is activated by pathogen-derived molecules allowing the body to detect unwanted pathogens early on as well as sense “danger” signals leading to the eventual destruction of those pathogens.
When RTKs are activated by an agonist, they form cross-linked dimers resulting in the activation of the tyrosine kinase by phosphorylation. Remember, kinases specifically act to catalyze the phosphorylation of a target molecule, which in this case is a neighboring RTK. The dimerized RTKs phosphorylate each other multiple times to result in signal amplification. This process is known as cross-phosphorylation. RTK cross-phosphorylation then leads to the phosphorylation of other proteins that will eventually result in modulation of gene transcription.
Key Pathway • Ras/RAF/Mitogen-Activated Protein [MAP] Kinase Pathway
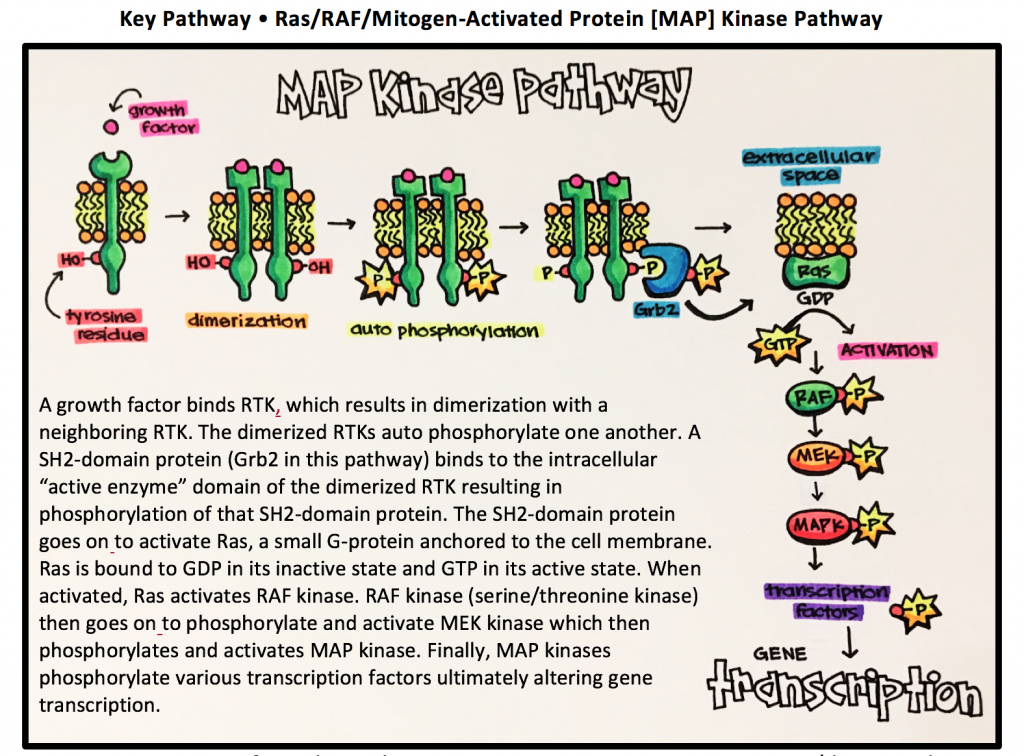
Ras = G-protein specific to this pathway RAF = proto-oncogene serine/threonine kinase
MEK = mitogen-activated protein kinase MAPK = mitogen-activated protein kinase
Ras • a small G protein (GTPase) involved in signal transduction leading to cell division and proliferation. If not regulated properly, Ras proteins can lead to uncontrolled cell division that eventually results in tumor formation.
RAF [Rapidly Accelerated Fibrosarcoma] • family of protein kinases that are involved with retroviral oncogenes (genes that can potentially cause cancer).
Tyrosine-Kinase Associated Receptors • associate with intracellular proteins that have tyrosine kinase activity. These receptors lack the tyrosine kinase domain that was discussed earlier and, therefore, accomplish tyrosine phosphorylation by cytoplasmic tyrosine kinases instead.
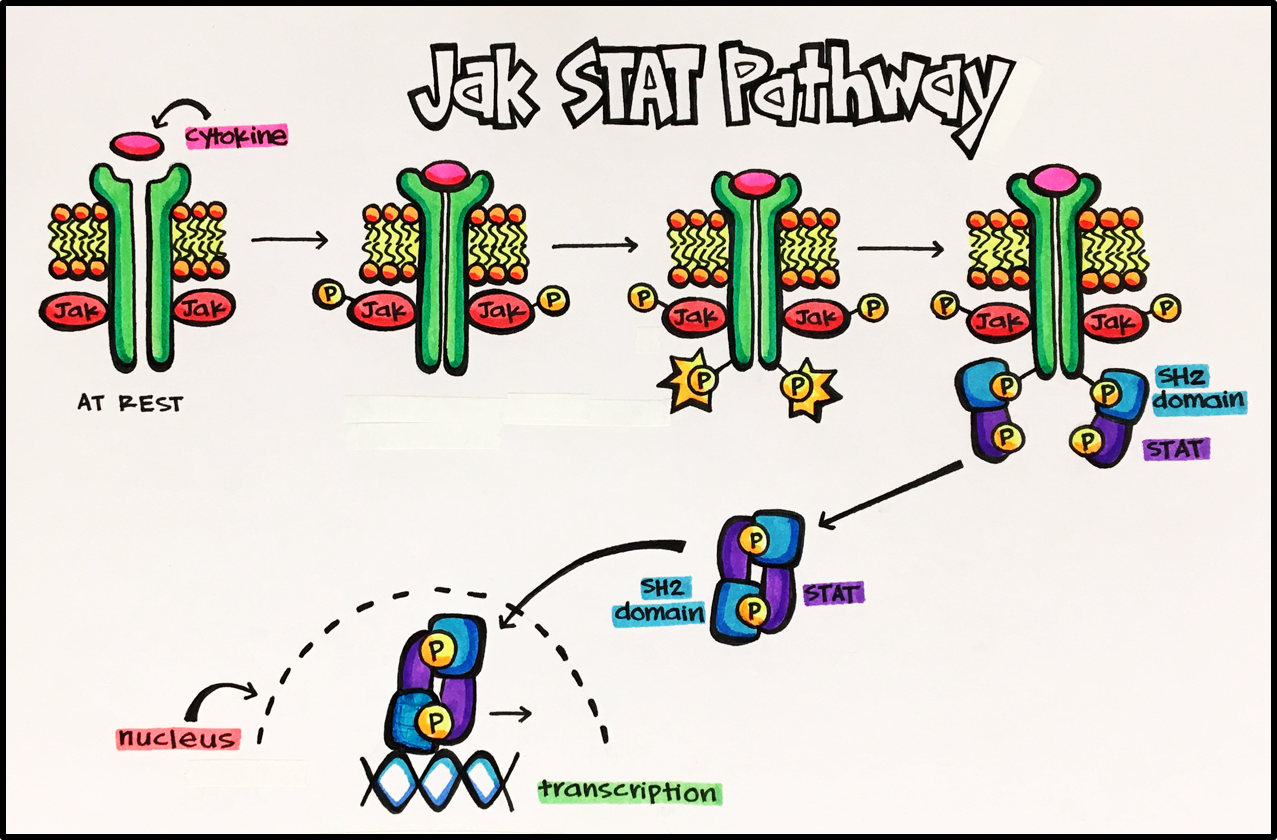
Cytokine receptors make up the largest family of receptors that relay signals into the cell by cytoplasmic tyrosine kinases. These particular receptors are associated with the cytoplasmic kinase, Jak (Janus kinase). Jak will go on to activate a gene regulatory protein called STAT (signal transducers and activators of transcription). The pathway is described and depicted below:
Key Pathway • Jak/Stat pathway (Janus kinase/signal transducers and activators of transcription) • The Jak/Stat pathway is the principal pathway for cytokines and growth factors in humans. This pathway is activated by a number of cytokines (most commonly interferons) and growth factors. Activation stimulates cell proliferation, differentiation, migration and apoptosis. Furthermore, cytokines control the synthesis and release of a number of inflammatory mediators. When a cytokine binds to its enzyme-linked receptor it results in a conformational change leading to phosphorylation of the intracellular active-enzyme domain, eventually leading to the transcription of inflammatory mediators. As with all signal transduction mechanisms, homeostasis is reliant on proper regulation of all these different pathways. Lack of proper regulation of the JAK pathway can cause inflammatory disease, erythrocytosis, gigantism and leukemias.
**For more information on enzyme-linked receptors, see this link below: