Building New Course Structures
5 Building Understanding and Appreciation: Collaborative Work in General Chemistry
Brian D. Gute
Keywords
flipped classroom, active learning, student teams, general chemistry
Challenges of Second-Semester General Chemistry
During my first semester of teaching at the University of Minnesota Duluth (UMD), I had the opportunity (some might call it a challenge) to teach both General Chemistry I (Chem1) and General Chemistry II (Chem2), the first and second semesters of the foundational chemistry course for science majors. As the semester progressed, I noticed that students in Chem2 struggled much more than the students in Chem1. When I mentioned this to some colleagues, they rationalized it as the difference between the “on-cycle” students in Chem1 and the “off-cycle” students in Chem2. After teaching the second-semester course multiple times, I recognize that in general there are differences in the preparation of so-called “on-cycle” and “off-cycle” students, however, the greater difference is in the complexity of the course content and the level of exposure that students already have to the material. A significant amount of material in Chem1 is covered in most high school chemistry courses, while the majority of students have never seen the material in Chem2. One unintended consequence is that many students who do well in the first-semester course rely in some part on their previous knowledge and do not learn the college study skills that they will need to succeed in more advanced courses.
Many students struggle in Chem2 because they are faced with completely new material, more challenging math (more complex algebra than needed in Chem1), and a faster pace than what they are used to when covering new material. As they do not realize that their study skills are not up to the task at hand, students find themselves struggling with multiple challenging factors all at the same time.
As I considered these issues, it became clear to me that if I wanted students to be more successful in Chem2, I needed to find ways to better support their learning, to help them develop better study skills, and begin to learn on their own. Over the course of the next five years, I read some of the scholarly literature on teaching, especially articles focused on chemistry education and active learning (Abraham, 2005; Bretz, 2005; Byers & Eilks, 2009; Cooper, 2005; Cracolice, 2005, 2009; Eberlein et al, 2008; Eilks et al, 2009; Farrell et al, 1999; Floriano et al, 2009; Gallagher-Bolos & Smithenry, 2004; Geiger et al, 2009; Greenbowe & Hand, 2005; Moog & Farrell, 2011; Moog et al, 2009; Spencer, 1999), and I experimented with a number of techniques designed to improve student engagement and success.
Could Flipping Address the Problem?
Ultimately, I wanted to change the way that I taught large lecture courses. While I enjoy lecturing, and feel energized after delivering what (in my mind) is a particularly engaging lecture, I am just as often disheartened by the questions I get after such “exceptional” lectures. It quickly becomes evident that my message was not as well received as I had imagined and that some of my students are just as confused about the material as they were at the beginning of the class period. I want my students to understand the fundamental chemical principles that we are covering and I want them to succeed in my class, but obviously I am not always getting through to them. Also, I believe that the human connection between the instructor and the student is an important component of motivation and learning, large lectures make it difficult to develop that connection. In large lecture settings, students become nameless faces amongst a sea of more nameless faces. I became convinced that if I was going to improve the experience for my students, I needed some way to connect with them as individuals. That connection would also provide me with a lens into their learning, giving me a clearer picture of what they understood and where they were still struggling.
In my efforts to innovate, I tried increasing the number of chemical demonstrations that I did in class, using active learning activities in their discussion sections and active learning techniques in lecture, including a student response system. While these changes helped, I did not see significant overall improvements. Often, the activities seemed to create more of a division within the class, helping the students who were already succeeding while doing little to nothing for the struggling students.
When I first heard about “flipped classes” I, like many of my colleagues, was skeptical. It sounded like the way college classes are supposed to work. Students come to class prepared, having read ahead in their books, and class time focuses on points of confusion and the more challenging aspects of the material. However, like other cynical academics, I am fairly certain that the majority of my students do not come to class prepared, and as a result, I feel compelled to cover all of the material since I know they are not covering it on their own.
Furthermore, I had a hard time picturing what a flipped class would look like. I had taken classes in college that were largely discussion-based, or spent equal amounts of time on lecture (to fill in needed background material) and discussion, but the vast majority of my experience with science courses was in a purely lecture format. I simply did not have the proper frame of reference to understand what a flipped class would look like and what we would do during class. At the same time there was something about the inclusion of technology in the learning process and the promise of more time to interact directly with my students that intrigued me enough that I started to delve deeper into the literature (and other resources) on flipped classes (Bergmann & Sams, 2012; Berrett, 2012; Bishop & Verleger, 2013; Educause Learning Initiative, 2012; Gimbar, 2011; Mangan, 2013; Musallam, 2011a-d; Neshyba, 2013; Smith, 2013; Sowash, 2012; Talbert, 2014a, 2014b).
In addition to my research, I participated in a faculty learning community on flipped classes and tried my hand at designing my own flipped lessons. I also experimented with process-oriented guided inquiry learning (POGIL) activities (Moog & Farrell, 2011) to get a sense of how students could spend class time constructing their understanding of the course content. For several years, my students were reluctant guinea pigs as I worked to better understand the ins-and-outs of the flipped classroom.
What I learned quickly was that the “flip” flopped if the instructor was not successful in getting the students to “buy-in” to this instructional approach, failed to hold the students accountable for the pre-class work (which is achieved by having some accountability measure or by reviewing the pre-class work at the beginning of class, thus inviting students to come prepared), and did not conduct the “flipped” lessons on a regular schedule, or as the standard approach to the course. To my mind, this last point was the most important. Flipped activities are very different from what students are familiar with and if they happen to infrequently, the students never get used to doing them. Every time you introduce another flipped lesson, there are challenges with getting the students to remember to do the pre-work and with getting them to willingly work in small teams. These challenges go away as soon as the flipped lessons begin to happen on a regular schedule and students know to plan for them.
I also devoted some of my time to seeking out “good” chemistry videos online, which was really an exercise in identifying what I did and did not like in “content-delivery” videos. There are many (in my opinion) really bad videos on Youtube and elsewhere, but I also found some gems with high production quality. Some of the best videos for general chemistry are the Crash Course Chemistry videos (Green, 2014) and the Khan Academy Chemistry videos (www.khanacademy.org/science/chemistry), though many of the Khan Academy videos are longer than I would like. Additionally, Nivaldo Tro has created some “Key Concept Videos” in support of his textbooks. Tro designed the videos to target key concepts that students tend to struggle with and they incorporate a final concept check question at the end. The Key Concept Videos can be accessed through Tro’s publisher, Pearson Education.
While exploring the flipped classroom approach, I also spent some of my time learning about the technologies that were available to help educators create videos. One of the most useful resources I stumbled across was a series of Youtube videos by Ramsey Musallam (2011a-d). These videos record a Youtube Teacher’s Studio course that he taught focused on the educational theory behind flipped teaching and the tools available to create videos in support of flipped teaching.
What I also learned was that preparing flipped lessons was time consuming. While one could simply record lectures based on existing lecture slides, the overwhelming message in the literature is that video “lectures” for flipped classes need to be short and to the point (Bergmann & Sams, 2012; Guo et al, 2014; Mangan, 2013; Mills, 1977; Musallam, 2011a-d; Pettis, 2015; Schwartz et al, 2009; Smith, 2013; Sowash, 2012). For me, this meant significantly reworking my existing lecture notes to make short segments emphasizing a specific concept or application in order to keep the videos at reasonable lengths (not more than 10–12 minutes, but preferably 5–8 minutes). Also, I needed activities to engage students with the concepts during class time for every flipped class period. Based on my experiences, I knew that if I was going to take the plunge, I wanted to flip the entire class rather than slowly work to incrementally build flipped materials. To make that happen, I would need some support.
At this point, I was not sure how I would manage the work involved in flipping the entire course, until several opportunities converged. I had already leveraged some small funding opportunities in my efforts to experiment with the approach and had acquired a writing capture tablet, a webcam for my desktop computer, a headset with microphone, and copies of Camtasia Studio and SnagIt software from TechSmith. These tools gave me better control over the quality (and editing) of my videos than the various free video creation tools and made it possible for me to create tutorial videos showing students how to work through mathematical problems using the writing capture tablet. Next, the new Dean of the Swenson College of Science and Engineering (SCSE) at UMD was promoting a shift within the college to widespread use of active learning techniques. And finally, a University of Minnesota system-wide call for Experiments in Learning Innovations (ELI) proposals came out from the Center for Educational Innovation (CEI) on the Twin Cities campus.
Making It Happen
At this point, I had all of the necessary tools to create “content delivery” videos for the course. The remaining challenges were to identify and create all of the necessary videos, organize my Moodle course site to support the flipped class approach, and develop the assessments that would hold students responsible for watching the videos in advance of class as well as the in-class activities that would replace lecture. Looking at this “short” list, I realized just what a monumental undertaking it would be to create a fully flipped course. In order to pull it off, I needed support from people who were well-versed in educational methods and the flipped classroom approach. The ELI request for proposals offered just that, access to educational experts from CEI and to educational technologists from the University’s Academic Technology Support Services (ATSS). With a successful proposal I might even have access to the resources necessary to create higher production quality videos than I would otherwise be able to make on my own.
In order to be competitive for the funding (and resources) from CEI, I proposed to do something that I had not seen in the literature, a direct comparison of lecture versus the flipped approach. This presented me with three challenges: traditionally, my Department only offers a single section of Chem2 in the fall; I wanted to make sure that the two sections I taught were roughly of the same size; and I wanted to teach the flipped class in a space with movable desks or tables to better facilitate team work. While my proposal was successful, I had some real challenges moving my vision for the project forward. UMD does not have many large classrooms that are not large lecture halls and rooms for the fall and spring had already been scheduled two months before it was announced that my project had been funded. This meant scrambling for whatever spaces might be available for the fall and ultimately limited the flipped class to a maximum enrollment of 54 students, while the lecture section enrollment was essentially unlimited since it was scheduled in a large lecture hall. Additionally, the creation of extra sections of the course in both the fall and spring semesters impacted my department’s budget and those costs had to be mitigated with funds from my budget for the project. That said, the ELI grant made the project possible, and provided me with a dedicated project manager to help me plan, identify available resources, and organize my work.
At this point, what I lacked was a plan for the overall structure of a totally flipped class. I knew that the “experts” all said that students need to be held accountable for doing the pre-class work, but how would I do that? And, how would I connect the accountability measure with the videos to create “packaged” pre-class work assignments that could be accessed on a single page of the LMS? These were all questions that I started to explore with my project manager as I started working on developing the course.
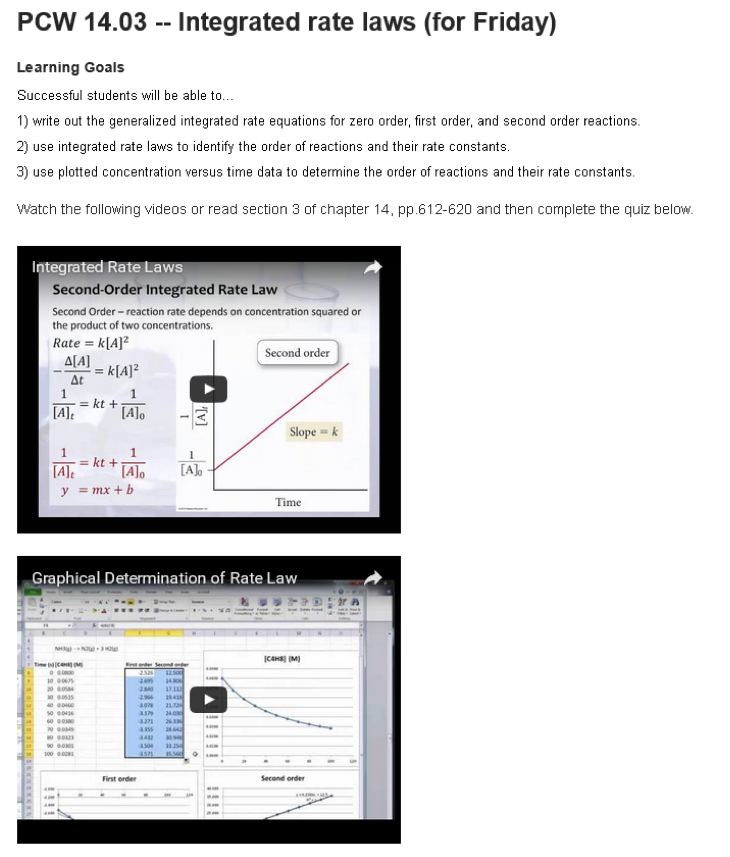 Figure 1: Example of a pre-class work (PCW) activity within the course Moodle site including learning goals, the alternate assigned reading, two embedded videos hosted on Youtube.
Figure 1: Example of a pre-class work (PCW) activity within the course Moodle site including learning goals, the alternate assigned reading, two embedded videos hosted on Youtube.
Designing and Managing Pre-Class Activities
With the guidance of my project manager, I started by writing specific student learning outcomes for each of the chapters to be covered and identifying topics that would require content videos. This daunting task led me to write 167 specific student learning outcomes and identify 63 unique topics that required videos. With that first task completed, I started to look for existing resources and identified 34 videos on Youtube and Khan Academy, leaving a minimum of 29 videos that I needed to create for the class. However, once I was done, I had a grand total of 65 videos, 31 of which I created using PowerPoint, a Surface Pro 3 (that I purchased to make writing capture easier), and Camtasia Studio. These new videos are hosted privately on Youtube and accessed through individual pre-class activities pages on the Moodle course site. The course site was rounded out with an additional five videos on relevant “topics of interest” intended to show students interesting applications of the concepts we were covering and three chemistry infographics from Compound Interest (www.compoundchem.com).
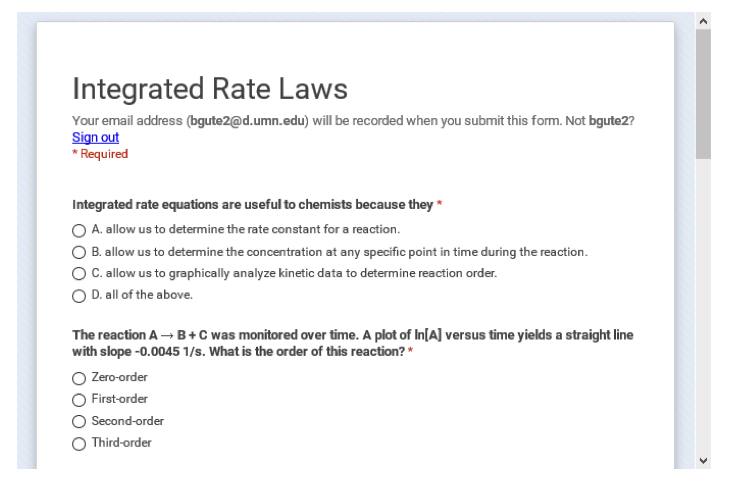
Figure 2: An example of the GoogleForm serving as a review quiz.
One of my goals in creating the pre-class activities (referred to as pre-class work or PCWs in class) was for all components of an activity to be available on the same page so students could see everything they needed to do to complete the assignment at a glance. After some discussion with my project manager, we agreed that each PCW should include a list of the student learning outcomes for the activity, the assigned videos (or the relevant pages within the textbook for students who preferred reading to watching videos), and a follow-up quiz to check for conceptual understanding and completion of the assignment. Because I wanted students to be able to access all three of these components for a given PCW in one place, each PCW resides on its own page in Moodle. This also meant that I could not use the quiz tool in Moodle since I could not create a quiz on a subpage and the quiz activity would take the students away from the page with the learning goals and the videos. Figure 1 presents an example of a PCW, with a list of specific student learning outcomes for the activity, a list of optional resources within the textbook that students could use (rather than the videos), embedded videos, and an embedded GoogleForm used as the quiz. I should mention that during the first two semesters of the flipped class, GoogleForms were just “dumb” surveys that collected data in a spreadsheet. I had to work within the spreadsheet to score the quiz after the deadline. As of late spring or early summer of 2016, a quizzing feature became available as part of the GoogleForm and made these GoogleForms much more useful for my class. Not only is the quiz self-scoring, but I can also set the quiz to show students which questions they answered incorrectly. In total, I created 38 PCWs for the fall semester of 2015, with some minor modifications for spring semester due to a textbook change.
Designing and Delivering In-Class Activities
I have to admit, when I proposed this project (and even into the first few months of work) I had not given much thought to what we would do in class, I was mostly focused on establishing learning outcomes for each of the chapters and the need to identify and create content videos. When I had given some thought to in-class activities, it was in very general terms. Further into the project it came time to start working on these activities, which I call in-class work activities (ICWs), and I really needed to do some thinking. While I dislike “worksheets” in college-level classes, I needed to give all of the students a set of problems/activities to spend time on in class.
It was during this stage of the project while I was discussing this issue with my project manager that I had the opportunity to sit in on Dr. Michelle Driesen’s flipped Chem2 course. Dr. Driesen’s students received their in-class activities in the form of electronic “worksheets” that they had to complete and that were graded on a regular basis. In all honesty, I was trying to avoid creating more work that would need to be graded (I was already planning to have graded homework), but quickly recognized that my students would need some incentive to complete the ICWs. After all, the whole point of the activities was for students to spend the time in class applying what they had learned from the videos while a teaching assistant and I were present to coach them through the work.
I needed a way to provide my students with these activities. I did not want to print out a new stack of activities for every class and I certainly did not want to regularly collect, grade, and redistribute a stack assignments. I needed a way to distribute and collect the activities electronically, and even grade and comment on the assignments electronically. I also wanted to have my students work with data and practice creating and interpreting graphs. I also wanted to be able to include images in the activities to promote visual thinking.
I had attended a few workshops and community of practice meetings at UMD where other faculty talked about using GoogleDocs to work collaboratively. All of our students have access to GoogleDrive and GoogleDocs, so using these electronic resources would only require some additional training and no additional cost for my students. At this point, my concept of working collaboratively in a GoogleDoc was still naïve (at best), but I knew that there was potential. Also, I could include full-color images in the GoogleDocs and if I shared the documents with my students, there would not be any reason to print the images/activities in color. Additionally, GoogleSheets would allow students to manipulate tabulated data and create graphs. This seemed like a solution that met all of my needs for the ICWs.
Each ICW is designed to accompany one of the pre-class assignments and the specific set of the student learning objectives for that PCW. Roughly 30-40% of each ICW focuses on conceptual material designed to help students build familiarity with the concepts and practice using the chemistry specific terminology to build their vocabulary. Generally, these conceptual sections then lead into specific applications, either creating or interpreting graphical representations of chemical data or practicing specific calculations that are central to applying or making predictions relevant to these concepts. One of my goals was to focus student attention on the foundational skills that they needed to be successful in the course, with a few more challenging application problems that often required them to work together and push their understanding a little further.
I initially started designing and creating ICWs with the assumption that we would need one for every day of class and I persisted in this delusional thought until my first set of students convinced me that some of my activities were far too long for a single 50-minute class period. At that point, I started to adjust my existing activities and design new ones based on the number of concepts to be covered per chapter and the depth to which we needed to cover any particular concept. All in all, this resulted in the creation of 40 in-class work activities for fall 2015.
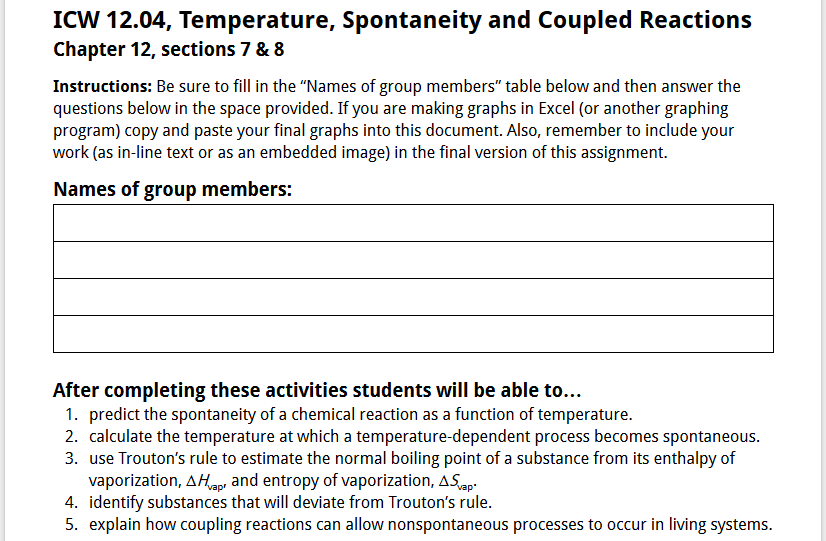
Figure 2: Example of the introductory section of an in-class work (ICW) for General Chemistry II.
To help students keep things in context, each ICW was titled and included the chapter and specific sections covered at the top of the activity. Additionally, each ICW includes a set of standard instructions, a table for including the names of the team members actively contributing to the work on the activity, and specific student learning goals related to the pre-class work and the in-class work (as shown in Figure 2).
Additionally, each ICW was divided into parts that covered specific concepts and those parts included three types of color-coded activities: Questions, Tasks, and Problems.
Figure 3 presents an example of a problem from an ICW. Note that problems were always color coded red and involved calculations. For every calculation problem, I included the numerical answer so students could quickly check their work to see if they had done the problem correctly. This was extremely helpful as it cut down on the number of questions about the “right” answer and allowed my teaching assistant and me to focus on helping students who were stuck or had made some mistake in their calculations. Questions (color coded purple) asked students to answer a specific conceptual question or provide a definition for an important term or concept. Finally, Tasks (color coded green) required students to do something else, such as graphing data points provided in the exercise and making specific observations related to the shape of the graph, or sketching and labeling a diagram illustrating a chemical process or important chemical apparatus.
One of the things I was most concerned about with online activities was having students show their work. After all, they should have a record of their work that they could return to for studying and since I was providing the answers to the problems, I wanted to emphasize the importance of the process of reaching the answer over simply the correct numerical value. The use of GoogleDocs greatly simplified this issue. Students could take a picture of their work using a cell phone or their laptop’s camera and embed the image in the GoogleDoc. And similarly, they could create graphs in GoogleSheets and copy and paste their work into the GoogleDoc.
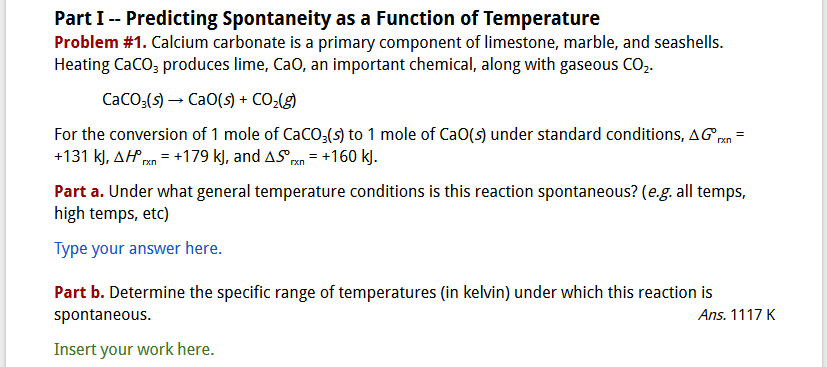
Figure 3: Example of a problem included in one of the in-class work (ICW) activity for General Chemistry II.
Successful implementation of the ICWs required trial and error. I had decided that students would work in teams of 3–4 and turn in a single copy of the ICW for their team. I initially hoped that I could give everyone access to a single copy of the GoogleDoc and have them all make their own copy, which was a huge disaster on the first day of class. Everyone, at essentially the same time, created a new file called “Copy of …” and almost everyone started working in that same document. It became clear that I needed to create a copy for each team. An ICW link from the Moodle site led to a shared GoogleDrive folder specific to that ICW, and the folder contained one copy of the ICW for each team. It might have been an inelegant solution, but it worked.
Who Needs to Manage Team Work? Apparently, I Do.
When I set out to flip Chem2, I knew that I had a lot of work to do to create all of the materials for the course. However, until I started creating the ICWs, I had not spent much time thinking about how I would organize class time. Similarly, I had not spent much time thinking about what would need to be graded, about whether students would work individually or in teams, or about classroom dynamics. Perhaps, subconsciously, I hoped that those issues would all take care of themselves while I was busy creating content for the course.
As I mentioned above, I eventually decided that students would work in teams. I made this choice for several reasons: 1) it would reduce the amount of grading that a teaching assistant or I would need to do; 2) students would be able to help each other learn the material; and 3) I was aware of some of the literature on the benefits of cooperative learning (Cooper, 2005; Eilks et al, 2009; Geiger et al, 2009). However, with so much of my attention focused on developing the materials and getting ready for my comparative study, I approached the creation of teams as a triviality and let my students pick their own teams. This had mixed results and the chaos of the first semester was heightened by the dysfunctionality of some of the teams. While some new friendships were formed that semester, I think others ended horribly.
When I mentioned this to my project manager, she was anything but surprised and simply asked me how many teams I had in total and how many of them were truly dysfunctional (in my lay assessment). When I told her that I had two truly dysfunctional teams (and perhaps one slightly dysfunctional team) out of eighteen total teams, she just looked at me and said, straight-faced, “That sounds about right.” Apparently, it is common for approximately 10% of teams to be dysfunctional. While that might be an accurate statistic, it did nothing to account for the fact that students in those teams were miserable and dealing with that drama, in addition to everything else was not doing much for me either.
That said, the class as a whole refused to change teams part of the way through the semester. And, in spite of the issues and the drama, in general the teams worked well together. One of the stranger dynamics that I witnessed during the first semester had to do with team-to-team interactions. We began the semester in a fairly traditional classroom with rectangular tables, and while there was a “front” to the room, I rarely used it as such. However, the students all sat facing the “front” of the room with team members sitting next to each other. While there were often members of two teams sitting side by side, there was rarely any cross talk between teams. Later in the semester, the class was moved into an “active learning” classroom with round tables that seated nine students each. Suddenly, two or three teams were forced to sit at the same table. The round tables allowed for easier interactions and the teams started to work collaboratively. This new collaboration between teams meant that students were helping each other even more, and my teaching assistant and I only needed to step in when the entire table misunderstood a concept or when the ICW problems became really challenging.
My teaching assistant noticed other interactions taking shape during the first semester. Always in the classroom before me, since my Chem2 lecture class was across campus and scheduled immediately before the flipped class, she noticed that most of the students arrived early and almost immediately started talking about chemistry, usually focusing on the videos or the ICW, but occasionally they were discussing how the class concepts related to other topics that interested them. These same interactions were observed during the second semester of the flipped class by a different teaching assistant. Going back to the team dynamics problem, obviously, I needed to do something with future classes to improve the team work experience for everyone. I once again turned to my project manager for advice, along with other colleagues who regularly use team work in their classes. They in turn gave me some useful suggestions and pointed me toward more of the literature on team work (Cooper, 2005; Dasgupta et al, 2015; Eilks et al, 2009; Geiger et al, 2009; Layton et al, 2010; Loughry et al, 2007; Loughry et al, 2014; Oakley et al, 2004; Ohland et al, 2012). I learned that: 1) I should be creating the teams, not allowing students to form their own teams; 2) long-term teams should be formed carefully, taking specific factors into account; 3) team work, when done well in STEM courses, can greatly benefit students from underrepresented groups (Dasgupta et al, 2015); and 4) ultimately the functioning of a team comes down to everyone agreeing to a set of shared expectations (Oakley et al, 2004).
This was all fairly overwhelming until I was pointed to the team formation tool (CATME Team-Maker), the peer evaluation tool (CATME Peer Evaluation) on the CATME SMARTER Teamwork website (www.catme.org), and the social contract creation resources within the manuscript by Oakley et al (2004). I implemented these tools in spring 2016 and had much better success with the teams. CATME Team-Maker helped me to form teams in which female students and students from other underrepresented groups were paired-up within the teams of three and to form teams with a range of academic levels (based on reported GPA and grade in the first-semester General Chemistry course). Once the teams were formed, each team was required to create a set of guiding principles that everyone in the team agreed to (a social contract) and to keep a copy for themselves and provide me with a copy as well. During this second offering of the flipped class, I only had issues with one team. That team had a late addition who was not present to participate in the creation of the team’s social contract and who was most likely was never told about the team contract by the other members of the team.
The current state of the flipped class
We are now well into the third iteration of Chem2 as a flipped class and the class has changed quite a bit. The first two semesters were relatively small sections, with 49 and 26 students respectively. Issues with the registration system and miscommunication about the course significantly limited enrollment in the spring of 2016 and marginalized my ability to get statistically meaningful results. Two flipped sections were offered in the fall of 2016 with initial enrollments of 90 students per section.
One challenge for me was that I needed to submit a proposal on moving forward with the flipped class approach during the 2016-17 academic year in November of 2015 – before I had any results from my study whatsoever. However, I had anecdotal information from discussions with the students and some practical experience with the class. One message my students sent me loud and clear was that the class periods needed to be longer. Several of the teams commented that they often felt like their team was just starting to “get it” and make really progress on an ICW when it was time to pack-up for the day.
In response to these comments, I proposed that we change the course from 50-minutes five times per week (four lecture periods and one discussion period) to 90-minutes twice per week, thinking that a 90-minute class period would give students plenty of time to work and would even make it possible to take a break during the middle of class. The 90-minute proposal was quickly shot down as a non-standard scheduling request and I was given the options for reducing class meetings of three 50-minute class periods per week or two 75-minute periods per week, which meant that I would effectively lose 100 minutes of class time per week.
My counter proposal was that I would make the two 75-minute class periods work so long as exams were conducted outside of normal class time, so that we did not lose even more instructional time throughout the semester. We settled on this schedule for the course, which meant that I needed to retool all of the activities to fit a two-day per week model instead of the five-days per week the class started with. And even though I have given up significant class time, I think it was worth it. The two-day per week model allows for a much more predictable schedule of assignments, quizzes, and exams, and students are more willing to finish their ICWs outside of class if they run out of time since they only come to class two days per week.
Team creation using CATME Team-Maker appears to work well and the team contracts have really helped to improve interactions within the teams. I know that in my section this fall (2016), out of 32 teams I have two or three teams that have some issues, but the issues are much less severe than those encountered during the fall semester and the students seem to have worked the issues out largely on their own. Oddly, from my perspective there have been more issues with students coming late to class or leaving early, but any time I have asked about it, the other team members tell me they know about the absence and have already had a conversation about how the missing member will contribute to the team’s work on that day’s ICW.
Of course, retooling the course to a two-day per week schedule meant combining or reconfiguring many of the PCWs and ICWs. I have also continued to create more videos and refine my existing videos, while also incorporating more “topic of interest” videos and additional infographics from Compound Interest (www.compoundchem.com). Table 1 summarizes how the number of “activities” associated with the course has changed over the course of three semesters.
Table 1: Summary of the number of activities, videos and infographics associated with the flipped course over three successive semesters.
| Fall 2015 | Spring 2016 | Fall 2016 | |
| Pre-class works (PCWs) | 38 | 34 | 27 |
| -Videos Created | 31 | 35 | 51 |
| -Other videos | 33 | 36 | 41 |
| In-class works (ICWs) | 40 | 34 | 27 |
| “Topic of interest” videos | 5 | 9 | 15 |
| Infographs | 3 | 7 | 7 |
Table 1 summarizes how the number of “activities” associated with the course has changed over the course of three semesters.Another substantive change has been the implementation of structured classroom facilitation and more standardized large group clarifications. One of the challenges I ran into during the first two semesters was keeping the teams “on track.” Some teams worked very efficiently, while others were not nearly as good at managing their time. Another challenge was that many student teams were uncomfortable with the open-ended conceptual questions in the ICWs and they sometimes blamed their poor performance on the conceptual exam questions on the fact that they never got the correct answers to those questions. Borrowing ideas from a workshop on facilitating POGIL activities (Schneider and Lovitt, 2016), I created short sets of slides for each class period that included how much time the teams would get to work on each section of the ICW and some follow-up questions for us to review as a class. I also added a final slide with daily reminders about activity deadlines. In addition to creating a more structured feel to the 75-minute class period and conveying to students how much time I thought they should need to complete each portion of the class activity, it helped to standardize what we were doing in class every day. This was especially important during the fall 2016 semester when I shared the teaching responsibility for the flipped class with a colleague. We were each teaching our own section of flipped Chem2, but the structured approach helped us to ensure that the student experience was essentially the same between both of the sections.
Lesson’s Learned
Flipping takes a lot of work. There is a lot of work involved in preparing for a semester-long, fully-flipped class. The prep work for this project required quite a bit of time during the preceding spring semester and summer and still required a significant amount of work during fall semester. Completely flipping your course all at once is not for the faint of heart.
Flipped classes are fun. While it was a lot of work, teaching the flipped class was the most fun I have had in my eight years of teaching. Instead of lecturing to students, I spent my time talking with students and answering their questions. It seemed like any time the work was starting to get to me, something happened in class that made my day and reminded me why I was putting so much work into this new class format.
Flipped classes can be fun for everyone. It’s a much less formal setting, students are constantly learning from each other, catching mistakes and misconceptions early on, and they are more comfortable asking each other and me for help when they get stuck. And, stepping into the classroom five minutes before class to find students already talking about chemistry is one of the coolest things I’ve experienced during my teaching career. It is really encouraging to see how engaged the students become with the material.
Clear communication is critical. Of course, there were learning opportunities for all of us. One of the most important lessons that I learned was the need to clearly communicate course expectations to the students. They are much more receptive to a new style of teaching if they have a clear understanding of what to expect from the very beginning. I was not as clear as I could have been with students in the fall 2015 section, and while they were good sports, they made it clear to me that they felt I had not been completely upfront with them about how class was going to work. Taking that lesson to heart, I spent more time talking to the students in the following two semesters and in each of those semesters, everyone had a much better understanding of what to expect from the class and as a result, I have had much less pushback from students related to the course format.
Less is more. By the end of the first month, it was also clear that five class periods per week with pre-class work (PCW), in-class work (ICW), and follow-up homework assignments for every day, or even most days, was too much for everyone to keep up with, including me. While I could not change the class schedule during the first two semesters, I did start to revise my approach to activities throughout the semester. I moved to fewer, but somewhat longer pre-class assignments and in-class activities designed to span several days. This reduced the number of days per week that students had required activities to complete outside of class.
This changed even more drastically in fall 2016 when the course switched to two days per week. The fact that there was a PCW and ICW for every class period and an associated homework assignment created a routine that students could plan around. It also removed any of the uncertainty from the first two semesters about when the next PCW or ICW was due.
Successful team work requires upfront preparation. Team dynamics need to be managed properly. I made some mistakes the first semester with how teams were formed and in not providing students with more guidance in setting team expectations. Giving students some choice, but creating assigned teams in a purposeful fashion in spring 2016 and fall 2016 led to much better results. Implementing team-developed social contracts for team expectations has also made a great improvement.
Relax and be flexible. Nothing is ever perfect the first time and most likely it still is not perfect the second (or even third) time either. If you feel that your class needs to run like a well-oiled machine and that you need to be in control of everything that happens in the classroom, a flipped class is not for you. More importantly with the flipped format, every team is going to be different, so you need to shift gears quickly to address issues and misunderstandings as they come up. Of course, this is also one of the strengths of the approach. We deal with misunderstandings as they arise to help students better learn the material. It is also much easier to see when students are struggling with the material.
Future Directions
The data from the first two semesters of this study are encouraging. The impact on the number of students withdrawing or ending the semester with D’s or F’s is incredibly exciting. In both the fall and spring semesters, the DFW rates (percentage wise) in the lecture section were twice the DFW rates in the flipped sections. Furthermore, attitude surveys conducted as part of the study show that students in the flipped class leave the course with a more positive opinion of chemistry in general, and that their experience with the course is also much more positive. With continued support from my Department and the Dean of SCSE, two flipped sections of Chem2 will be offered this spring (2017) and in the fall and spring of the next academic year (2017-18). Out of necessity, the flipped sections will continue to have enrollments of 90 to 100 students per section, but based on experiences during fall 2016, I know that this is manageable with sufficient instructional support from teaching assistants.
References
Abraham, M.R. (2005). Inquiry and the Learning Cycle Approach. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching. (pp 41–52). Upper Saddle River, NJ: Pearson Education, Inc.
Bergmann, J., & Sams, A. (2012). Flip Your Classroom: Reach Every Student in Every Class Every Day. Eugene, OR: International Society for Technology in Education (iste).
Berrett, D. (2012). How ‘Flipping’ the Classroom can Improve the Traditional Lecture. In A Guide to the Flipped Classroom. (2-6). The Chronicle of Higher Education, http://www.chronicle.com/resource/a-guide-to-the-flipped-classro/5882/ [viewed 28 Nov 2016].
Bishop, J.L., & Verleger, M. (2013). The Flipped Classroom: A Survey of the Research. In Proceedings, 120th American Society for Engineering Education Annual Conference & Exposition, https://peer.asee.org/the-flipped-classroom-a-survey-of-the-research [viewed 28 Nov 2016].
Bretz, S.L. (2005). All Students are not Created Equal: Learning Styles in the Chemistry Classroom. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching. (pp 28–40). Upper Saddle River, NJ: Pearson Education.
Byers, B., & Eilks, I. (2009). The Need for Innovations in Higher Level Chemistry Education – A Pedagogical Justification. In I. Eilks & B. Byers (Eds.), Innovative Methods of Teaching and Learning Chemistry in Higher Education. (pp 5–22). Cambridge: The Royal Society of Chemistry.
Cooper, M.M. (2005). An Introduction to Small-Group Learning. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching. (pp 117–128). Upper Saddle River, NJ: Pearson Education, Inc.
Cracolice, M.S. (2005). How Students Learn: Knowledge Construction in College Chemistry Courses. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching. (pp 12–27). Upper Saddle River, NJ: Pearson Education, Inc.
Cracolice, M.S. (2009). Guided Inquiry and the Learning Cycle. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching: Volume II. (pp 20–34). Upper Saddle River, NJ: Pearson Education, Inc.
Dasgupta, N., McManus Scircle, M., & Hunsinger, M. (2015). Female Peers in Small Work Groups Enhance Women’s Motivation, Verbal Participation, and Career Aspirations in Engineering. Proceedings of the National Academy of Science of the United States of America, 112(16), 4988-4993.
Eberlein, T., Kampmeier, J., Minderhout, V., Moog, R.S., Platt, T., Varma-Nelson, P., & White, H.B. (2008). Pedagogies of Engagement in Science: A Comparison of PBL, POGIL, and PLTL. Biochemistry and Molecular Biology Education, 36(4), 262-273.
Educause Learning Initiative (ELI). (2012). 7 Things You Should Know About Flipped Classrooms. https://library.educause.edu/resources/2012/2/7-things-you-should-know-about-flipped-classrooms [viewed 28 Nov 2016].
Eilks, I., Markic, S., Bäumer, M., & Schanze, S. (2009). Cooperative Learning in Higher Level Chemistry Education. In I. Eilks & B. Byers (Eds.), Innovative Methods of Teaching and Learning Chemistry in Higher Education. (pp 103–122). Cambridge: The Royal Society of Chemistry.
Farrell, J.J., Moog, R.S., & Spencer, J.N. (1999). A Guided Inquiry General Chemistry Course. Journal of Chemical Education, 76, 570-574.
Floriano, M.A., Reiners, C.S., Markic, S., & Avitabile, G. (2009). The Uniqueness of Teaching and Learning Chemistry. In I. Eilks & B. Byers (Eds.), Innovative Methods of Teaching and Learning Chemistry in Higher Education. (pp 23–41). Cambridge: The Royal Society of Chemistry.
Gallagher-Bolos, J.A., & Smithenry, D.W. (2004). Teaching Inquiry-Based Chemistry: Creating Student-Led Scientific Communities. Portsmouth, NH: Heinemann.
Geiger, L., Jones, L., & Karre, I. (2009). Transforming Lecture Halls with Cooperative Learning. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching: Volume II. (pp 49–70). Upper Saddle River, NJ: Pearson Education, Inc.
Gimbar, K. [Lodge McCammon]. (2011, May 2). Why I Flipped My Classroom Retrieved from https://youtu.be/9aGuLuipTwg
Green, H. [CrashCourse]. (2014, Jan 13). Crash Course Chemistry Retrieved from https://youtu.be/FSyAehMdpyI?list=PL8dPuuaLjXtPHzzYuWy6fYEaX9mQQ8oGr
Greenbowe, T.J., & Hand, B. (2005). Introduction to the Science Writing Heuristic. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching. (pp 140–154). Upper Saddle River, NJ: Pearson Education, Inc.
Guo, P.J., Kim, J., & Rubin, R. (2014). How Video Production Affects Student Engagement: An Empirical Study of MOOC Videos. In Proceedings of the First ACM Conference on Learning @ Scale Conference (L@S ’14). (pp 41-50). New York: ACM.
Layton, R.A., Loughry, M.L., Ohland, M.W., & Ricco, G.D. (2010). Design and Validation of a Web-Based System for Assigning Members to Teams Using Instructor-Specified Criteria. Advances in Engineering Education, 2 (1), 1-28.
Loughry, M.L., Ohland, M.W., & Moore, D.D. (2007). Development of a Theory-Based Assessment of Team Member Effectiveness. Educational and Psychological Measurement, 67, 505-524.
Loughry, M.L., Ohland, M.L., & Woehr, D.J. (2014). Assessing Teamwork Skills for Assurance of Learning Using CATME Team Tools. Journal of Marketing Education, 36(1), 5-19.
Mangan, K. (2013). Inside the Flipped Classroom. In A Guide to the Flipped Classroom. (7-9). The Chronicle of Higher Education, http://www.chronicle.com/resource/a-guide-to-the-flipped-classro/5882/ [viewed 28 Nov 2016].
Mills, H.R. (1977). Techniques of Technical Training, 3rd edition. London: MacMillan.
Moog, R.S., & Farrell, J.J. (2011). Chemistry: A Guided Inquiry. Hoboken, NJ: John Wiley & Sons, Inc.
Moog, R.S., Creegan, F.J., Hanson, D.M., Spencer, J.N., Straumanis, A., Bunce, D.M., & Wolfskill, T. (2009). POGIL: Process-Oriented Guided-Inquiry Learning. In N.J. Pienta, M.M. Cooper & T.J. Greenbowe (Eds.), Chemists’ Guide to Effective Teaching: Volume II. (pp 90–107). Upper Saddle River, NJ: Pearson Education, Inc.
Musallam, R. [teach’s channel]. (2011a, Sep 15). Ramsey Musallam: Flipteaching – Part 1 Retrieved from https://youtu.be/rub1VNq2NvM
Musallam, R. [teach’s channel]. (2011b, Sep 15). Ramsey Musallam: Flipteaching – Part 2 Retrieved from https://youtu.be/FIwKVRQJ1PE
Musallam, R. [teach’s channel]. (2011c, Sep 15). Ramsey Musallam: Flipteaching – Part 3 Retrieved from https://youtu.be/jjElGeJaHZc
Musallam, R. [teach’s channel]. (2011d, Sep 15). Ramsey Musallam: Flipteaching – Part 4. Retrieved from https://youtu.be/9MzxczsF9bA
Neshyba, S. (2013). It’s a Flipping Revolution. In A Guide to the Flipped Classroom. (10-12). The Chronicle of Higher Education, http://www.chronicle.com/resource/a-guide-to-the-flipped-classro/5882/ [viewed 28 Nov 2016].
Oakley, B., Felder, R.M., Brent, R., & Elhajj, I. (2004). Turning Student Groups Into Effective Teams. Journal of Student Centered Learning, 2(1), 9-34.
Ohland, M.W., Loughry, M.L., Woehr, D.J., Finelli, C.J., Bullard, L.G., Felder, R.M., Layton, R.A., Pomeranz, H.R., & Schmucker, D.G. (2012). The Comprehensive Assessment of Team Member Effectiveness: Development of a Behaviorally Anchored Rating Scale for Self and Peer Evaluation. Academy of Management Learning & Education, 11(4), 609-630.
Pettis, T. (2015, Jan 13). Flipping with Kirch: Designing a Flipped Learning Environment Retrieved from https://www.sophia.org/tutorials/flipping-with-kirch-webinar-011315
Schneider, J., & Lovitt, C. (2016). The POGIL Project Workshop: Classroom Facilitation, Presented at the 2016 Biennial Conference on Chemical Education (BCCE), Greeley, CO, July 31-August 4, 2016.
Schwartz, M.S., Sadler, P.M., Sonnert, G., & Tai, R.H. (2009). Depth Versus Breadth: How Content Coverage in High School Science Courses Related to Later Success in College Science Coursework. Science Education, 93(5), 798-826.
Smith, J.D. (2013). Student Attitudes Toward Flipping the General Chemistry Classroom. Chemical Education Research and Practice, 14, 607-614.
Sowash, J. [GoEd Online’s channel]. (2012, Apr 23). 5 Things I Wish I Knew When I Flipped My Class Retrieved from https://youtu.be/4JPdGlyt6gg
Spencer, J.N. (1999). New Directions in Teaching Chemistry. Journal of Chemical Education, 76, 566-569.
Talbert, R. (2014a). Toward a Common Definition of ‘Flipped Learning’. In A Guide to the Flipped Classroom. (13-14). The Chronicle of Higher Education, http://www.chronicle.com/resource/a-guide-to-the-flipped-classro/5882/ [viewed 28 Nov 2016].
Talbert, R. (2014b). Flipped Learning Skepticism: Do Students Want to Have Lectures? In A Guide to the Flipped Classroom. (15-17). The Chronicle of Higher Education, http://www.chronicle.com/resource/a-guide-to-the-flipped-classro/5882/ [viewed 28 Nov 2016].
Acknowledgements
I would like to take the opportunity to thank the University of Minnesota Center for Educational Innovation for supporting my Educational Learning Innovations research project and, especially, Kris Gorman, J.D. Walker, and Paul Baepler for their invaluable assistance with the design and analysis of the project. I would also like to thank the members of the first Swenson College of Science and Engineering Active Learning Cohort for their friendship and support throughout this endeavor, and Drs. Bilin Tsai, Elizabeth Austin-Minor, and Joshua Hamilton for their confidence in me and support, both personal and administrative, throughout this grand experiment.

