1.2 Hyperadrenocorticism – Canine
Learning Objectives
- Know that 85% of the spontaneous hyperadrenocorticism (HAC) cases are caused by a functional pituitary tumor (i.e. pituitary dependent) and, 15% are caused by an adrenal tumor (i.e. adrenal dependent).
- Know that the common historical signs associated with HAC are polyuria, polydpsia, polyphagia, panting, exercise intolerance and hair loss.
- Know the organ systems affected in HAC and the characteristic clinical signs.
- Understand the pathomechanism of polyuria, polydpsia, polyphagia, muscle wasting, and pot-belly in patients with HAC.
- Understand the pathomechanism of panting in an animal with HAC.
- Understand the pathomechanism of hepatomegaly in an animal with HAC.
- Understand the pathomechanism of proteinuria in an animal with HAC.
- Know and understand the abnormalities present in the hemistry profile and urinalysis of a dog with HAC.
- Remember! The ACTH stimulation test is used to diagnose HAC not to differentiate pituitary-dependent hyperadrenocorticism (PDH) from adrenal-dependent hyperadrenocorticism (ADH). This test evaluates the adrenal reserve of cortisol.
- Remember! The accuracy of the ACTH stimulation test is about 85% if a dog has PDH but of only about 50% if a dog has ADH.
- Remember! The ACTH stimulation test is the gold-standard test to diagnose iatrogenic HAC.
- Know and understand the results of an ACTH stimulation test in a normal dog, a dog with spontaneous HAC, and a dog with iatrogenic HAC.
- Remember! The low-dose-dexamethasone-suppression test can diagnose HAC in about 95% of the cases. Therefore, it is more accurate diagnostic test than the ACTH stimulation test. However, it cannot be used to differentiate spontaneous from iatrogenic HAC.
- Know and understand the results of the low-dose-dexamethasone-suppression test in a normal dog and a dog with HAC.
- Remember! The low-dose-dexamethasone-suppression test can differentiate PDH from ADH in approximately 60% of the cases. Be able to interpret the test results in these cases.
- Know the other tests used to differentiate PDH from ADH and how to interpret these tests.
- Remember! Dogs with diseases other than HAC can have ACTH and low-dose-dexamethasone-suppression test results similar to the results of a dog with HAC. In other words, false positive results can occur.
- Know how to manage PDH. Be sure to understand well the protocols of trilostane and mitotane therapy.
- Know the side effects associated with trilostane and mitotane therapy. Learn how to manage cases that develop side effects caused by these drugs.
- Know the HAC cases that are candidates for ketoconazole therapy.
- Know how to manage adrenal tumors.
-
General Considerations
- Canine hyperadrenocorticism (HAC) or Cushing’s disease is considered a common endocrinopathy.
- The syndrome is caused by persistently high concentrations of cortisol in circulating blood.
- HAC can be spontaneous or iatrogenic.
- Spontaneous HAC is classified as pituitary-dependent or adrenal-tumor dependent.
- Spontaneous HAC is a disorder of dogs over 6 years of age and over 75% of dogs with spontaneous HAC are older than 9 years.
- Iatrogenic HAC may occur in dogs of any age or breed and it is caused by prolonged oral, topical, or parenteral administration of glucocorticoids.
- Many nonadrenal diseases cause clinical signs that overlap with those seen in dogs with HAC (such as renal failure, diabetes mellitus, liver disease, pyelonephritis, hypothyroidism, diabetes insipidus, and side effects of anticonvulsant drugs). Therefore, a complete evaluation of the patient for nonadrenal diseases is recommended before a diagnosis of hyperadrenocorticism is made.
Important Facts
- Hyperadrenocorticism (HAC) can be spontaneous or iatrogenic.
- The spontaneous form is classified as pituitary-dependent or adrenal-tumor dependent and is usually seen in dogs older than 6 years of age.
- The iatrogenic form is caused by prolonged administration of glucocorticoids and it can occur at any age.
- Many nonadrenal diseases cause clinical signs that mimic HAC. Therefore, a complete evaluation of the patient for nonadrenal diseases is recommended before a diagnosis of HAC is made.
-
Pathogenesis
- The cause of Pituitary-dependent hyperadrenocorticism (PDH) is a functional pituitary tumor (i.e. either a microadenoma (most common) or macroadenoma).
- In this case the pituitary secrets excessive amounts of adrenocorticotrophic hormone (ACTH) resulting in bilateral adrenocortical hyperplasia, which leads to excessive production of cortisol.
- PDH accounts for 80% to 85% of the spontaneous cases.
- Adrenal-dependent hyperadrenocorticism (ADH) is caused by a functional adrenocortical tumor. This form of HAC accounts for about 15% to 20% of the spontaneous cases.
- Functional adrenal tumors can be adenocarcinomas or adenomas and unilateral or bilateral tumors. In most cases the tumor is unilateral.
- When unilateral, the contralateral adrenal gland becomes atrophied as a result of the negative feedback suppression of ACTH caused by the excessive cortisol production by the tumor.
- Iatrogenic HAC caused by inappropriate glucocorticoid treatment results in bilateral atrophy of the adrenal cortex. This form may represent more than half of the cases.
Important Facts
- Pituitary-dependent hyperadrenocorticism (PDH) is caused by a functional pituitary tumor.
- It accounts for 80% to 85% of the spontaneous cases of HAC.
- Pituitary microadenomas are more common than macroadenomas.
- The excessive production of ACTH by the pituitary tumor will induce bilateral hyperplasia of the adrenal cortexes resulting in excessive production of cortisol.
- Adrenal tumor accounts for about 15% to 20% of the spontaneous cases of HAC.
- It is usually caused by a unilateral adenoma or adenocarcinoma but the tumor can be bilateral.
- In the case of unilateral tumors, the contralateral gland is atrophied due to feedback suppression of ACTH secretion from excessive cortisol production by the tumor.
- Iatrogenic HAC may account for more than half of the HAC cases.
-
About the disease
- Signalment:
- Spontaneous HAC is most commonly seen in middle age to older dogs. More than 75% of dogs with PDH and more than 90% of dogs with ADH are older than 9 years of age (range 2-18 years., mean 10-11 years).
- There is no sex predilection.
- The disease is seem most commonly in Poodles, Dachshunds, Beagles, Boxers, Boston Terriers, and German shepherd dogs.
- Common historical findings:
- Polyuria (80% – 85%) – The pathomechanism is not well understood but most investigators believe that cortisol in excess interferes with the secretion of antidiuretic hormone (ADH) by the posterior pituitary (i.e. central diabetes insipidus). Other possible causes of polyuria include: (i) cortisol interference of ADH action on the distal tubules and collecting duct cells (i.e. nephrogenic diabetes insipidus); (ii) cortisol increase of the glomerular filtration rate (GFR) by causing vasodilation of afferent arterioles; therefore, increasing renal blood flow and GFR, initiating diuresis; and rarely, (iv) compression of the posterior pituitary or hypothalamus or hypothalamic stalk by a large anterior pituitary tumor (macroadenoma), which ultimately interferes with the secretion of ADH.
- Polydipsia (80% – 85%) – Secondary to the polyuria (i.e. compensatory).
- Polyphagia (90%) – It is believed to be a direct effect of excess glucocorticoids stimulating hunger in the dog.
- Lethargy and muscle weakness – The catabolic effect of cortisol on protein ultimately results in muscle wasting and weakness.
- Painting – Dogs with HAC often present with increased respiratory rate at rest (tachypnea). Possible explanation for this clinical sign include (i) fat accumulation in the thoracic cavity, (ii) wasting and weakness of the muscles involved in respiration (catabolic effect of glucocorticoids on protein), (iii) compression of diaphragm by an enlarged liver and abdominal fat accumulation, (iv) pulmonary interstitial mineralization, which interferes with oxygen perfusion and blood oxygenation resulting in hypoxemia, and (v) significant decrease in expiratory reserve volume.
- Weight gain – Most of the time the weight gain is apparent. The fat redistribution and distended abdomen (potbellied appearance) give an impression of weight gain.
- Dermatologic abnormalities – Non-inflammatory alopecia, thin skin, hyperpigmentation, etc.
- Clinical signs:
- Pendulous abdomen (potbelly): occurs in 90%-95% of the cases and is more easily noted in chronic disease. Many factors contribute to this common sign:
- Abdominal fat accumulation.
- Hepatomegaly – Chronic circulation of excess cortisol causes hepathopathy associated with glycogen accumulation in periportal cells and centrolobular hepatocyte vacuolization resulting in an enlarged liver.
- Urinary bladder distention.
- Abdominal muscle wastage leading to muscle weakness, which results from the catabolic effects of cortisol. The dog in the picture has a distended abdomen (i.e. potbelly appearance) because of abdominal muscle wastage due to the catabolic effect of cortisol primarily on proteins.
- Pendulous abdomen (potbelly): occurs in 90%-95% of the cases and is more easily noted in chronic disease. Many factors contribute to this common sign:
- Signalment:
-
-
- Generalized muscle weakness attributed to the muscle wastage that result from the catabolic effects of excess cortisol on proteins.
- Respiratory signs:
- Panting – See above under “Common historical findings”.
- Pulmonary thromboembolism due to a hypercoagulable state present in some dogs with HAC. It is a more serious complication of HAC, which may produce acute and severe dyspnea.
- Dermatological signs:
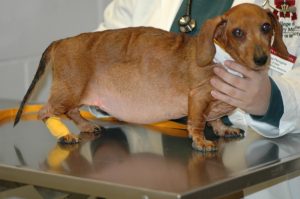
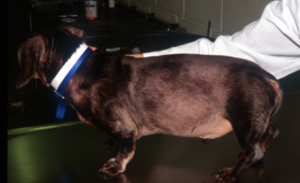
- Alopecia (non-inflammatory) – It usually has a bilateral and symmetrical pattern. It spares the head and distal extremities. The pictures below show the classical pattern of non-inflammatory hair loss in dogs with HAC. Distended abdomen can also be seen in both dogs.
-
-
-
-
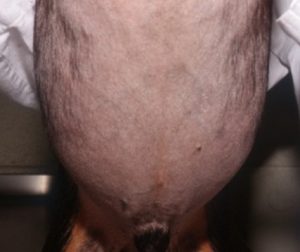
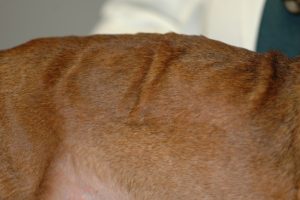
- The skin can become hypotonic because of the catabolic effect of cortisol in the skin elastic fibers. The picture below shows skin folds that do not regress because of the loss of skin elasticity in a dog with advanced HAC.
-
-
-
-
-
- Noticeable abdominal vasculature because of thin skin, which occurs from the catabolic effect of cortisol on protein, fat and carbohydrate.
-
-
-
-
-
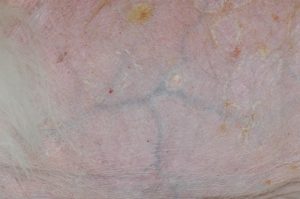
- The picture shows stria on the left aspect of the abdomen and bilaterally at groin because of decreased skin elasticity. The picture also shows a distended abdomen.
-
-

-
-
-
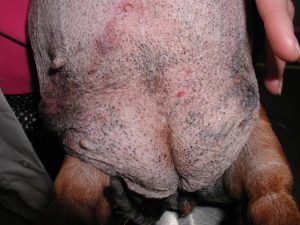
- Various comedones (“black heads”) distributed along the ventral abdomen of a dog with HAC.
-
-
-
-
-
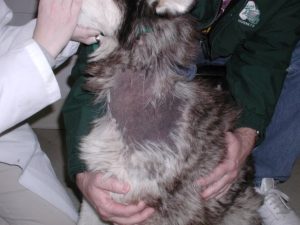
- Hyperpigmentation of the skin occurs frequently in patients with HAC.
-
-
-
-
-
- Easy bruising due to fragility of blood vessels.
- Poor wound healing.
-
-
-
-
-
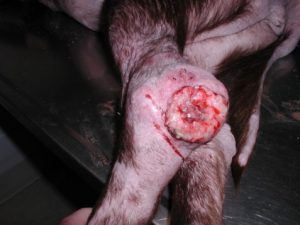
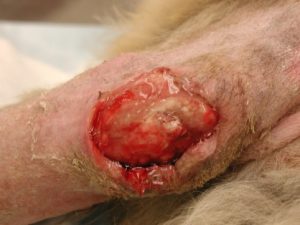
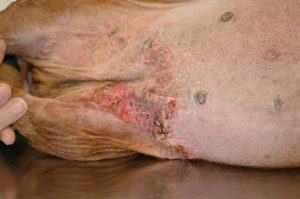
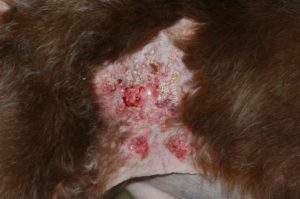
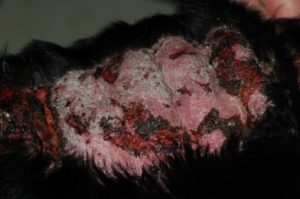
- Calcinosis cutis occurs in about 40% of the cases and can manifest in different ways. The lesion typically presents as erythematous to whitish plaques that are firm to the touch and can ulcerate. A pale tightly adhered scale/crust eventually forms on the surface and often represents elimination of the dermal calcium through the epidermis. When the plaque is ulcerated, the crust is typically black. It usually involves the neck, ventral abdomen, and/or inguinal area. However, it can develop anywhere in the skin including the perianal and interdigital regions.
-
-
-
-
-
- Recurrent superficial pyoderma that can manifest as large bullous pustules develop in some cases.
-
-
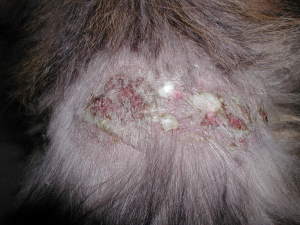
-
-
-
- Complications such as, adult onset demodicosis and dermatophytosis rarely occur.
- Seborrhea sicca clinically manifested as excessive fine scales is a common clinical sign.
- Medical complications:
- Many dogs with HAC are not severely ill but the chronic exposure to excess cortisol can result in complications that are sometimes severe. We will discuss four medical complications associated with canine HAC: hypertension, diabetes mellitus, blindness, and urinary tract infections.
- Hypertension – About 50% of randomly tested dogs with HAC will have hypertension. Cortisol and cortisone have a weak mineralocorticoid activity and will increase Na+ absorption in the kidney and increase K+ and H+ secretion, which will result in extracellular fluid volume expansion and hypertension. In addition, glucocorticoids increase the responsiveness of arterioles to catecholamines by up-regulating alpha 1 –adrenergic receptors. Cortisol and glucocorticoids in general reduce the synthesis of vasodilators, such as prostaglandins, and increase concentrations of endothelin-1, a potent vasoconstrictor secreted by the endothelium.
- Diabetes mellitus – Diabetes mellitus only occurs in about 5%-10% of dogs with HAC. Cortisol and glucocorticoids in general have a potent gluconeogenic effect and increase glucose synthesis in the expense of protein and fat. They have a catabolic effect on protein and reduce the amino acid transport to muscle cells (reduce protein synthesis) but increase the amino acid transport to liver cells resulting in increased synthesis of glucose from amino acid. Glucocorticoids also increase lipolysis resulting in an increase of glycerol and free fatty acids. Glycerol will be utilized in the liver to produce glucose. Finally, glucocorticoids decrease glucose utilization by tissues and decrease the sensitivity of adipocytes to insulin.
- Blindness: Blindness may occur secondary to hypertension and retinal detachment. Large pituitary tumors (i.e. macroadenomas) may cause blindness but usually also cause concurrent neurologic signs such as somnolence, disorientation, ataxia, and seizures.
- Urinary tract infections (UTI) – Dogs with HAC are prone to develop UTI and often do not present clinical signs. The predisposition to develop UTI can be explained by the anti-inflammatory effect of cortisol and glucocorticoids in general, which inhibits the migration of neutrophils and macrophages to sites of infection and by the diluted urine associated with HAC (refer to polyuria above).
- Many dogs with HAC are not severely ill but the chronic exposure to excess cortisol can result in complications that are sometimes severe. We will discuss four medical complications associated with canine HAC: hypertension, diabetes mellitus, blindness, and urinary tract infections.
-
-
Important Facts
- The 5 Ps: Polyuria, Polydipsia, Polyphagia, Panting and Potbelly are common clinical signs of HAC.
- Lethargy and exercise intolerance are common complaints and are usually associated with muscle wastage caused by the catabolic effect of glucocorticoids on proteins.
- Common dermatologic signs are symmetrical non-inflammatory alopecia, thin skin, poor wound healing, prominent abdominal vasculature, easily bruised skin, dystrophic calcinosis cutis, and recurrent superficial pyoderma.
- Less common medical complications include hypertension, diabetes mellitus, blindness and UTI.
-
Diagnosis
- Characteristic historical findings.
- Characteristic clinical signs. Remember, some nonadrenal disorders have history and clinical signs that overlap with those seen in dogs with HAC, especially the polyuria and polydipsia.
- Minimal database must be acquired to determine the presence of nonadrenal disorders and to determine the nature and extent of clinicopathologic abnormalities that exist in the patient. The minimum data base should include:
- Complete blood cell count.
- Serum chemistry profile.
- Urinalysis.
- Urine culture.
- Clinicopathologic abnormalities associated with HAC:
- Stress leukograma, which include neutrophilia, monocytosis, lymphopenia, and eosinopenia.
- Elevated serum alkaline phosphatase (SAP) induced by glucocorticoids is present in about 80% to 95% of dogs with HAC. Values can exceed 1000 IU/L and do not correlate with the severity of HAC, response to therapy, or disease prognosis.
- Alanine aminotransferase can also be mildly elevated due to hepatocellular leakage associated with cell swelling, glycogen accumulation, interference with hepatic blood flow or hepatocellular necrosis.
- Hyperglycemia may result from increased gluconeogenesis as well as insulin antagonism by glucocorticoids.
- Hypercholesterolemia and lipemia are believed to be the result of lipolysis.
- Usually, urine specific gravity is low in about 85% of the cases (dilute urine, < 1.020).
- Glycosuria and diabetes mellitus have been reported to occur in about 5% of the cases.
- Proteinuria occurs in 44% to 75% of dogs with HAC and should be quantitated with urine protein:creatinine ratio (mean ratio = 2.3; some cases = 5-13). Proteinuria is speculated to be the result of hypertension that is also commonly seen in dogs with HAC. Hypertension can result in glomerulopathy.
- Specific diagnostic tests:
- Urine cortisol:creatinine ratio – This test is used to rule out HAC.
- It is a sensitive test (~75-100%) for detection of increased urinary cortisol excretion.
- Unfortunately, this test is not specific for diagnosing HAC (~20-25%). Seventy-five to 80% of dogs with nonadrenal diseases also have increased urine cortisol:creatinine ratio.
- It has a negative predictive value close to 100%; that is, the likelihood that the test correctly predicts that a dog does not have HAC is close to 100%.
- Think about performing this test when you want to rule out HAC. If the test is negative, you can feel confident that there is a very small chance that the dog has HAC. However, if the test is positive you have to perform an ACTH stimulation test or low-dose-dexamethasone suppression test before confirming the diagnosis of HAC.
- Protocol:
- To avoid the effect of stress, owners should collect two urine samples at least 2 days after a visit to a veterinary hospital/clinic.
- The morning urine sample is preferred because it usually represents several hours of urine production.
- ACTH stimulation test – This test is used to diagnose spontaneous and iatrogenic HAC.
- The ACTH stimulation test is designed to evaluate the capacity of the adrenal glands to secrete cortisol after maximal stimulation. In other words, it evaluates the cortisol reserve in the adrenal glands.
- When to perform the ACTH stimulation test?
- To distinguish between spontaneous and iatrogenic HAC. This is currently the only test that can accomplish it.
- To diagnose spontaneous HAC. The ACTH stimulation test can diagnose PDH in about 80% of the cases and ADH in only 50% to 60% of the dogs. However, some authors argue that the ACTH stimulation test can only diagnose about 60% of the PDH cases of HAC.
- To monitor response to therapy with o, p’–DDD (Lysodren®, Mitotane®) or trilostane (Vetoryl®). Normal dogs show moderately increased cortisol levels after ACTH administration. Baseline cortisol level ranges from 1.5 to 6.0 µg/dl (15 to 60 ng/ml) in normal dogs; the post-ACTH cortisol levels vary from 8.0 to 16 µg/dl (80 to 160 ng/ml) in normal dogs.
- Spontaneous HAC cases show markedly increased response to ACTH compared to normal dogs (post-ACTH cortisol levels > 20 µg/dl [>200 ng/ml]). This response is expected because the adrenal glands reserve of cortisol in dogs with HAC is significantly higher compared to normal dogs.
- Iatrogenic HAC shows minimal or no increase in cortisol levels after ACTH administration because the adrenal cortex is atrophied resulting in minimal reserve of cortisol. This occurs due to the negative feedback of the exogenous glucocorticoids at the corticotrophs in the pituitary gland, inhibiting the secretion of ACTH.
- The test does not differentiate dogs with PDH from those with ADH.
- The ACTH stimulation test may be abnormal (false-positive) in dogs with nonadrenal illness but this is seen less often than the low-dose dexamethasone suppression test.
- A diagnosis of HAC should not be confirmed or excluded based only on the ACTH test result because this test is associated with false-negative and false-positive results. Therefore, nonadrenal diseases (may cause a false-positive result) need to be ruled out first and the history and clinical signs have to support a positive test result. If the test is negative but the history and clinical signs are characteristic of HAC, perform a low-dose dexamethasone suppression test.
- Remember that cortisol cross-reacts with prednisone, prednisolone, methylprednisolone, hydrocortisone, fludrocortisone and cortisone. Therefore, make sure to discontinue any of these glucocorticoids for at least 24 hours before performing the ACTH stimulation test to avoid spuriously high serum cortisol concentration and a false-positive result.
- More than one protocol have been described for the ACTH test:
- Fasting is not needed before performing the test but because the effect of feeding in the ACTH stimulation test result is unknown, it is recommend to not feed the animal during the test.
- Because dogs do not have a circadian cortisol secretion, the ACTH stimulation test can be started at any time during the day.
- Synthetic ACTH has greater purity and quality control than compounded products; therefore, the use of compounded ACTH is discouraged.
- Various studies have shown that Cortrosyn™ (cosyntropin), cosyntropin injection (only for IV use) and Synacthen® (tetracosactrin) can be used interchangeably.
- The ACVIM published a consensus statement in 2013 (see reference below) and the panel recommended the following protocol:
- Administer 5 µg/kg IV of the preferred compound (listed above), and collect blood samples before and 60 minutes after administration.
- Urine cortisol:creatinine ratio – This test is used to rule out HAC.
Important Facts
- The ACTH stimulation test is a quick and moderately reliable test for diagnosing HAC.
- It is the only reliable test to differentiate iatrogenic from spontaneous HAC.
- The ACTH stimulation test can diagnose PDH in about 80% of the cases and ADH in only 50% to 60% of the dogs.
- False-positive results can occur in cases of nonadrenal illnesses.
- A diagnosis of HAC should not be confirmed or excluded based on the ACTH test results only.
- The ACTH stimulation test result should be interpreted in the context of the patient’s history, clinical signs, and clinicopathologic test results.
- This test does not differentiate dogs with PDH from those with AT.
-
-
- Low-dose-dexamethasone-suppression test (LDDST) – This test is used to diagnose and localize the disease.
- To diagnose spontaneous HAC.
- It has a sensitivity of 85% – 97%, so it is more reliable than the ACTH stimulation test.
- In approximately 95% of dogs with PDH and 100% of dogs with ADH, cortisol levels at 8 hours do not suppress below 1.4 µg/dl (14 ng/ml [some laboratories will have slightly different cutoff values]). However, in about 5% of dogs with PDH, the plasma cortisol level suppresses normally.
- In normal dogs, the cortisol level is suppressed to < 1.4 µg/dl (14 ng/ml) at 8 hours.
- To differentiate between PDH and ADH.
- In approximately 60% to 65% of the PDH cases, the 4-hour post-dexamethasone sample suppresses the plasma cortisol level to any of the following values: (i) lower than 50% of the baseline value, (ii) lower than 50% of the value at 8 hours, or (iii) bellow 1.0 to 1.4 µg/dl.
- If no significant suppression is observed at 4 hours, the LDDST will not be able to differentiate between PDH and ADH (this occurs in about 35% to 40% of the cases).
- In approximately 60% to 65% of the PDH cases, the 4-hour post-dexamethasone sample suppresses the plasma cortisol level to any of the following values: (i) lower than 50% of the baseline value, (ii) lower than 50% of the value at 8 hours, or (iii) bellow 1.0 to 1.4 µg/dl.
- The LDDST can be positive in dogs with nonadrenal illness (false-positive results); therefore, interpret the test in the context of a characteristic history and clinical signs and rule out nonadrenal diseases that have similar signs.
- Remember that cortisol cross-reacts with prednisone, prednisolone, methylprednisolone, hydrocortisone, fludrocortisone and cortisone. Therefore, make sure to discontinue any of these glucocorticoids for at least 24 hours before performing the LDDST to avoid spuriously high serum cortisol concentration and a false-positive result.
- Protocol:
- Fasting is not needed before performing the test but because the effect of feeding in the LDDST result is unknown, it is recommend to not feed the animal during the test.
- Because dogs do not have a circadian cortisol secretion, the LDDST can be started at any time during the day.
- Either 0.01 mg/kg or 0.015 mg/kg of dexamethasone sodium phosphate or dexamethasone in polyethylene glycol is administered intravenously after a blood sample has been obtained for determination of baseline plasma cortisol levels. Samples are collected again at 4 and 8 hours after administration of dexamethasone. If dexamethasone sodium phosphate is used, calculate dose using a concentration of 3 mg/mL, instead of 4 mg/mL as listed on the bottle.
- The 4-hour post-dexamethasone blood sample is used to help differentiate between PDH and ADH.
- Because cortisol concentrations vary by assay and between laboratories, it is important to not change laboratories when trying to diagnose a patient.
- To diagnose spontaneous HAC.
- Low-dose-dexamethasone-suppression test (LDDST) – This test is used to diagnose and localize the disease.
-
Important Facts
- The LDDST test diagnoses PDH in about 95% of the time and ADH in 100% of the time.
- If a dog has either PDH or ADH, the 8-hour post-dexamethasone cortisol levels should be 1.4 µg/dl (14 ng/ml) or higher.
- The LDDST test can distinguish PDH from ADH in about 60% of the cases.
- If the 4-hour post-dexamethasone cortisol level is below 50% of the baseline value or below 50% of the value at 8 hours, or if the 4-hour level suppresses bellow 1.0 or below 1.4 µg/dl, it indicates a case of PDH. However, if no suppression occurs at 4 hours, the dog can have either PDH or ADH (i.e. no differentiation can be made).
-
-
- High-dose-dexamethasone-suppression test (HDDST) – This test is used only to localize the disease.
- The HDDST test is only used to differentiate between PDH and ADH.
- The ACTH hormone production by pituitary tumors is suppressed by negative feedback if a large enough dose of dexamethasone is used (i.e. 10 times the dose used in the LDDST), and, as a result, the plasma cortisol level is also suppressed.
- The high dose of dexamethasone does not suppress the plasma cortisol level if the dog has an ADH (remember, the adrenal tumor secretes cortisol independently of the pituitary gland).
- If a dog has PDH:
- The 4-hour post-HDDST cortisol concentration has to be < 1.4 µg/dL or < 50% of the baseline cortisol concentration, which indicates suppression.
- The 8-hour cortisol concentration is less than 50% of the baseline concentration.
- Lack of suppression may occur in as many as 15% to 25% of dogs with PDH, necessitating the use of another discrimination test. Thus, the sensitivity of this test to differentiate between PDH and ADH is low making it a less than ideal test.
- If a dog has ADH:
- The 4-hour or 8-hour post-HDDST cortisol concentration will not suppress more than 50% of the pre or baseline value as adrenal tumors secrete cortisol independently of the pituitary gland.
- When the serum cortisol level is not suppressed during the HDDST test, a different test should be performed to localize the disease.
- Protocol:
- Intravenous administration of 0.1 mg/kg (i.e. 10 times the dose used in the LDDST) of dexamethasone sodium phosphate or dexamethasone in polyethylene glycol is recommended. If dexamethasone sodium phosphate is used, calculate dose using a concentration of 3 mg/mL, instead of 4 mg/mL as listed on the bottle.
- Blood samples for measurement of plasma cortisol levels are collected before and 8 hours after dexamethasone administration.
- High-dose-dexamethasone-suppression test (HDDST) – This test is used only to localize the disease.
-
Important Facts
- The HDDST test is used to differentiate between PDH and ADH.
- It fails to differentiate PDH from ADH in 15% to 25% of the time.
- If a dog has PDH (pituitary tumor), the high dose of dexamethasone will suppress the pituitary ACTH and consequently decrease the plasma cortisol concentration to less than 50% of the baseline value.
- If a dog has an ADH, the 4-hour or 8-hour post-HDDST cortisol concentration will not suppress more than 50% of the pre or baseline value as adrenal tumors secrete cortisol independently of the pituitary gland. In this case-scenario, a different test will be required to localize the disease.
-
-
- Endogenous ACTH – This test is used only to localize the disease.
- Like the HDDST, the endogenous ACTH test is only used to differentiate between the PDH and ADH.
- In dogs with ADH, the ACTH secretion is suppressed by negative feedback of cortisol. In contrast, excessive ACTH secretion is associated with PDH.
- Reference ranges vary according to the technique used and each laboratory must establish its own reference ranges.
- Sample handling:
- Blood samples must be handled quickly because the ACTH half-life is only 25 minutes in fresh whole blood because plasma proteases degrade endogenous ACTH rapidly.
- The blood should be collected in chilled plastic tubes because ACTH adheres to glass. If collecting in glass tubes, the EDTA tubes should be silicone coated to avoid adherence of the ACTH to the glass.
- The tubes should be immediately centrifuged (within 15 minutes and ideally in a cooled centrifuge) and frozen in plastic tubes for transport to the laboratory in frozen condition.
- If samples are shipped, they should be packed in dry ice and sent overnight.
- An in-house test, TruForma benchtop endocrine analyzer (Zomedica), measures endogenous ACTH concentration allowing for prompt analysis of the hormone without the need to prepare the sample as described above.
- Endogenous ACTH – This test is used only to localize the disease.
-
Important Facts
- Endogenous ACTH concentration is used to differentiate PDH from ADH.
- Reference ranges vary according to the technique used and each laboratory must establish its own reference ranges.
- If samples are shipped, they should be packed in dry ice and sent overnight.
-
-
- Abdominal radiography, abdominal ultrasonography, computed tomography or magnetic resonance imaging:
- Abdominal radiography – It is used to localize the disease.
- In one report, 56% of 23 dogs with ADH had radiographic evidence of adrenal calcification.
- Because adrenal calcification is rare in normal dogs and dogs with PDH, its presence in a dog with HAC suggests ADH and most likely associated with an adenocarcinoma (adenomas generally do not mineralize).
- Abdominal ultrasonography – It is used to localize the disease.
- In most dogs with ADH, the tumor is large enough to be visualized by a trained ultrasonographer.
- The hyperplastic adrenal glands associated with PDH can be visualized by ultrasound in most cases. However, measurements can overlap between normal dogs and dogs with HAC. In addition, dogs with nonadrenal illnesses may have hyperplastic adrenal glands to the extent of dogs with PDH. Therefore, the finding of a normal size or enlarged adrenal glands with normal shape in a dog diagnosed as having HAC is considered strong evidence of PDH. On the other hand, in a dog diagnosed with HAC, a finding of a single adrenal gland that is abnormally shaped and that may be compressing or invading adjacent structures (such as, vena cava, liver, or kidney) in association with a contra-lateral gland that is small, thin, or not visualized is suggestive of a functioning adrenocortical tumor.
- Other function of the abdominal ultrasound in a dog with HAC is the evaluation of the liver, masses within any organ, presence of urinary calculi etc.
- Ultrasound should never be used as a tool to diagnose HAC; it is use to differentiate PDH from ADH cases!
- Computed tomography (CT) or magnetic resonance imaging (MRI):
- These diagnostic tools have been used more frequently to diagnose pituitary tumors larger than 8 to 10 mm in greatest diameter.
- If expense and anesthesia risk are not concerns, a CT scan or MRI should be considered as a prognostic factor in any dog diagnosed with PDH. The following is recommended:
- If a dog with PDH has no visible mass (< 3 to 4 mm), unlikely the pituitary tumor will grow to an extent to cause clinical signs. A repeat scan is not recommended for such cases.
- If a dog has a visible mass (7 mm in greatest diameter), medical therapy for HAC is recommended and the scan should be repeated 12 to 24 months after the first scan. If the mass has increased 2 to 4 times in size, brain radiation therapy is recommended.
- If a dog has a pituitary mass of 8 mm in greatest diameter, radiation therapy is warranted before starting medical therapy for HAC. Nevertheless, these dogs will also need medical therapy.
- Abdominal radiography – It is used to localize the disease.
- Abdominal radiography, abdominal ultrasonography, computed tomography or magnetic resonance imaging:
-
Important Facts
- Abdominal radiography can show calcification of the adrenal gland in cases of functional adrenal tumors (i.e. ADH).
- Because adrenal calcification is rare in normal dogs and dogs with PDH, its presence in a dog with HAC suggests ADH; however, keep in mind that the sensitivity of this test to diagnose ADH is low.
- Abdominal ultrasonography may provide information that helps distinguish PDH from ADH and may show abnormalities of other abdominal organs.
- Pituitary CT and MRI scans are useful to investigate the size of the tumor and make recommendations regarding performing future scans or the need for radiation therapy of the brain.
-
Treatment
- Pituitary-dependent hyperadrenocorticism (PDH):
- Trilostane (Vetoryl®, Dechra)
- FDA approved for the treatment of canine HAC – 5 mg, 10 mg, 30mg, 60mg and 120 mg capsules. Avoid using compounded formulations because of large variability in efficacy between batches, which may result in variable efficacy of the product.
- It is a competitive inhibitor of the 3 ß-hydroxysteroid dehydrogenase enzyme system blocking the synthesis of cortisol and, to a lesser extent, aldosterone. Its inhibitory effect is reversible.
- Rarely adrenocortical necrosis occurs in dogs treated with trilostane.
- Various studies have documented the efficacy of trilostane in managing canine PDH cases.
- The dosage should be tailored for each individual patient. Studies have shown that twice daily administration can result in better clinical response compared to once daily as trilostane has a relatively short-lasting effect. Because of potential side effects, it is recommended to start the dose at 1.0–2.0 mg/kg twice daily depending on the FDA-approved commercial available capsule sizes. Dose may need to be re-adjusted based on response to therapy and drug tolerance of each patient. Trilostane should be given with food to increase absorption.
- Response to therapy is monitored with ACTH stimulation tests or measurements of pre-pill serum cortisol concentrations.
- ACTH stimulation test monitoring: Perform the test in 10-14 days, then 1 month later, and then every 3 to 6 months. It is important to call the client weekly during the first month to make sure the patient is tolerating the medication well. If side effects of hypocortisolemia are noted, the pet owner should not give another trilostane dose and an ACTH stimulation test should be performed immediately. Perform the ACT stimulation test at 3 to 5 hours after the morning administration of trilostane. Be consistent on the timing to perform the ACTH stimulation tests in each patient. This time corresponds to the maximal effect of trilostane. Test results and, more importantly, the patient’s clinical signs will dictate if the dose will need to be adjusted.
- During monitoring of treatment response, the ACTH dose can be 1 µg/kg IV.
- Maintain the post-trilostane ACTH stimulating test timing consistent between visits. For example, if the first test was performed at 4 hours after the trilostane dose, perform all future tests at 4 hours post-trilostane dose.
- If the patient is receiving unequal doses twice daily (e.g. 40 mg in the morning and 30 mg in the evening), perform the ACTH stimulation test after the higher dose (e.g. 40 mg).
- If the post-ACTH cortisol levels are lower than 1 µg/dl stop the medication for 48 hours and resume therapy with the next lowest capsule size.
- If the post-ACTH cortisol level is higher than 5.0 µg/dl, increase the dose to the next highest capsule size.
- If the post-ACTH cortisol levels are between 1.0 and 5.0 µg/dl, serum electrolytes are normal and the dog appears clinically normal, continue the dosage as is. If the animal is unwell (i.e. lethargic, reduced appetite or anorexic etc.) and the serum electrolytes are abnormal (i) discontinue treatment for a few days, (ii) start the patient on 0.1-0.2 mg/kg/day of prednisolone or 0.01-0.02 mg/kg/day of dexamethasone until signs resolve, which should happen quickly, and, (iii) re-start treatment at the next lowest dose.
- Remember, these numbers are given as guidelines and pay more attention to the patient than the numbers. For example, if the post-ACTH cortisol level is over 5.0 µg/dl but the patient is doing well and clinical signs are improving, dose adjustment is not warranted.
- Some clinicians prefer to perform the ACTH stimulation test between 8 to 12 hours after the morning dose when administering the medication twice daily. In a study, the mean post-ACTH cortisol level 9 hours post pill in well-controlled dogs receiving trilostane twice daily was 8.1 ± 3.4 µg/dL. The recommended upper limit of the ideal range when performing the test at 8-12 hours after the morning dose is 9-14 µg/dL. The advantage of performing the ACTH stimulating test 8-12 hours post pill is to determine if the patient is not at risk of hypocortisolemia just before the next trilostane dose.
- The goal of the first recheck visit at 14-days following initiation of trilostane therapy is to determine if the serum concentration of cortisol is not too low. Do not increase the trilostane dose at that time, if the serum concentration of cortisol is relatively high. If needed, increase the dose at the following visit as it may take a while for the drug effect to be noted. However, reduce the dose if the cortisol level is too low.
- Pre-pill serum cortisol concentration monitoring – Keep in mind that the most important factor to take into consideration when treating HAC is the patient’s clinical signs independent of the drug or protocol you adopt.
- Start trilostane at 1.0 to 2.0 mg/kg every 12 hours.
- Recheck in 10-14 days. At this visit, perform a thorough history and physical examination. If the animal is doing well continue the same treatment regimen. If the animal is not feeling well (i.e. somewhat lethargic, appetite is reduced etc.), measure serum electrolytes and perform an ACTH stimulation test (3-5 hours post pill). Discontinue therapy for 5-14 days if the test results support hypoadrenocorticism. Start the patient on 0.1-0.2 mg/kg/day of prednisolone or 0.01-0.02 mg/kg/day of dexamethasone until signs resolve, which should happen quickly. Re-start therapy at the next lowest capsule size. If signs persist, reconsider other differentials. If the animal is doing well and the pre-pill cortisol level is above 5.0 µg/dl do not increase the dose at this early visit even if the signs of HAC have not improved as it may take a few weeks for improvement to be noted.
- Recheck in 28-30 days.
- If no clinical signs of HAC and the pre-pill cortisol level is 1.0 to 5.0 µg/dl, continue the current dose and schedule a recheck visit in 3 months. If the pre-trilostane cortisol level is <1.0 µg/dl but the patient is well, signs of HAC are improving and electrolytes are unremarkable, continue with the same treatment regimen and schedule a recheck in 30 days. If the patient is unwell and electrolytes are abnormal, perform an ACTH at 8-12 hours after the morning dose and consider reducing the dose to the next lowest trilostane capsule size.
- If the patient has signs of HAC and the pre-pill cortisol level is ≥1.0-1.5 µg/dl give the dose every 12 hours if not already doing so or increase the dose to the next highest capsule size. If the pre-pill cortisol level is <1.0-1.5 µg/dl and the patient has clinical signs of HAC, consider ruling out other diseases or give more time for the trilostane to work and recheck in 30 days. If considering a dose increase, make sure to perform an ACTH stimulation test to ensure adequate post-stimulation cortisol levels (i.e. adequate adrenal cortisol reserve).
- Trilostane (Vetoryl®, Dechra)
- Pituitary-dependent hyperadrenocorticism (PDH):
Important Facts
- Trilostane has been shown to be an excellent alternative to o, p’-DDD in the treatment of canine HAC.
- Its effect is reversible and side effects should resolve shortly after drug discontinuation.
- Start with a low dose (e.g. 1.0 to 2.0 mg/kg every 12 hours) and adjust the dose based primarily on the patient’s clinical response but also on electrolytes values and results of an ACTH stimulation test or pre-trilostane cortisol concentration.
- Recheck the patient in 10-14 days, then 30 days later and every 3 to 6 months thereafter. However, maintain close communication with the client. If the patient is not doing well, schedule a recheck visit immediately.
-
-
- Lysodren® (o,p’-DDD):
- It effectively reduces cortisol secretion by causing a selective necrosis and atrophy of the zona fasciculata and zona reticularis of the adrenal cortex. These zones are responsible for the production of cortisol and sex hormones.
- The zona glomerulosa (mineralocorticoid production) is relatively resistant to o,p-DDD. Therefore, mineralocorticoid deficiency associated with hyperkalemia and hyponatremia are uncommon (about 6%-10% of the cases).
- Because Lysodren® does not cure the disease, dogs require weekly lifelong therapy.
- Lysodren® therapy is divided into two phases: the induction and the maintenance.
- Induction phase:
- The induction phase consists of “loading” Lysodren® with the objective of returning the dog’s levels of cortisol to the normal range.
- General recommendation: 40 to 50 mg/kg q 24h, orally, for 7 to 10 days or less if signs of cortisol deficiency (lethargy, depression, weakness, vomiting, diarrhea) occur. Divide the daily dose to twice a day administration and feed the animal before administering the Lysodren®. Do not administer the dose if the dog does not eat and instruct the owner to bring the dog in for an ACTH stimulation test and/or chemistry profile.
- Lysodren® should be given with a fatty food to increase absorption.
- Send owners home with prednisone/solone. If the dog shows signs of cortisol deficiency (lethargy, depression, weakness, vomiting, diarrhea), instruct the client to contact the veterinarian and supplement the dog with oral prednisone/lone at the dose of 0.1-0.2 mg/kg/day or 0.01-0.02 mg/kg/day of dexamethasone until signs resolve, which should happen quickly. If no improvement is noted after 1 to 3 hours, the animal has to be examined immediately and a chemistry profile should be performed to rule out mineralocorticoid deficiency (hyperkalemia and hyponatremia). In addition, Lysodren® therapy should be discontinued and an ACTH stimulation test performed to assess the degree of adrenal cortex atrophy.
- If no signs of mineralocorticoid or glucocorticoid deficiency are noted, the ACTH stimulation should be performed in 7 to 10 days to assess the response to Lysodren® therapy.
- If the post-ACTH cortisol levels are within or slightly below the normal range (such as, 20 to 40 ng/dl or 2 to 4 µg/dl), a maintenance dosage of Lysodren® may be started.
- If prednisone, prednisolone or methylprednisolone is given at any time during Lysodren® therapy, be sure to stop glucocorticoid administration for 24 to 48 hrs before having an ACTH stimulation test performed because glucocorticoid will cross-react with cortisol resulting in a spuriously high serum cortisol level.
- Maintenance phase:
- A maintenance dosage of Lysodren® involves giving 50 mg/kg every 7 days. However, divide this weekly dose and give it two to three times during the week.
- Lower dosages may result in an increased incidence of relapse.
- Concurrent glucocorticoid supplementation generally is not necessary.
- An ACTH stimulation test should be repeated after 1 to 2 months of maintenance therapy and every 6 months thereafter. However, perform the test sooner if signs of hypocortisolemia develop or clinical signs of HAC return.
- If signs of HAC return and cortisol levels rise above the normal range, an induction or loading phase should be reinstituted, and an ACTH stimulation test should be performed in 7 to 10 days or sooner if signs of hypocortisolemia develop. Another option if relapse occurs is to increase the weekly maintenance dosage by 50% to 100%.
- Approximately half the dogs treated with Lysodren® relapse within 12 months.
- Lysodren® (o,p’-DDD):
-
Important Facts
- Lysodren® (o,p’-DDD) causes necrosis and/or atrophy of the adrenal cortex resulting in the reduction of cortisol secretion.
- It rarely destroys the zona glomerulosa, so mineralocorticoid deficiency (hyperkalemia and hyponatremia) rarely occurs.
- The induction phase consists in giving Lysodren® daily for about 7 to 10 days or less if signs of hypocortisolemia develop.
- The maintenance phase consists in giving the daily induction dose of Lysodren® weekly, divided in 2-3 doses.
- Give Lysodren® with a fatty meal to increase absorption.
- An ACTH stimulation test should always be performed before starting the maintenance phase to assess the response to the daily Lysodren® administration.
- An ACTH stimulation test should be performed after 1 to 2 months of the maintenance therapy and every 6 months thereafter.
- Stop prednisone/solone or methylprednisolone therapy 24 to 48 hours before having an ACTH stimulation test performed because they cross-react with cortisol, which may result in a spuriously high serum cortisol concentration.
- The most common side effects associated with Lysodren® therapy include lethargy, vomiting, diarrhea, weakness, anorexia, and ataxia, which indicate induction of hyadrenocorticism.
-
-
- Ketoconazole:
- It is an antifungal medication but it also decreases cortisol levels.
- It interferes with adrenal steroid synthesis by blocking enzymes in the cortisol-synthesis pathway.
- Ketoconazole should be tried when dogs cannot tolerate o.p-DDD or trilostane, owners cannot afford these drugs, or they are not commercially available. It can also be used in dogs with adrenal neoplasia with high risk for surgery or with metastasis.
- The initial dosage is 5 mg/kg q 12h for 7 to 10 days. Ketoconazole has to be given with food to assure adequate absorption.
- Treatment success is monitored with an ACTH stimulation test after 7 to 10 days of therapy. If the post-ACTH cortisol levels are within the desired range, maintain the same dose and repeat the test in 30 days. Thereafter, perform the test every 3 to 6 months for the first year and then once a year.
- If the post-ACTH cortisol levels are above the desired range and clinical signs have not improved, increase the dose to 10-15 mg/kg q 12h and repeat the ACTH stimulation test in 7-10 days.
- The ACTH stimulation test should be performed 1 to 3 hours after ketoconazole administration. It is not necessary to discontinue ketoconazole before testing.
- Failure to respond to dosages as high as 15 mg/kg q 12h occurs in up to 20% of cases. However, a study showed that 90% of dogs with PDH had evidence of clinical improvement when treated with ketoconazole. The dosage ranged from 5 to 25mg/kg given twice daily (median 12.5mg/kg twice daily). Improvement was noticeable in most cases after two months of therapy.
- Reported side effects include anorexia, vomiting, and a lightening of the hair coat. Increase in liver enzymes and hepatotoxicity can also occur.
- Lifelong therapy twice daily is necessary because the drug blocks the adrenal steroid synthesis without destroying adrenal tissue.
- Ketoconazole:
-
Important Facts
- Ketoconazole interferes with adrenal steroid synthesis by blocking enzymes in the cortisol-synthesis pathway, therefore, lowering serum cortisol concentrations.
- Success rate is variable and ketoconazole should only be tried when dogs cannot tolerate o.p-DDD and trilostrane, these drugs are not available commercially, or owners cannot afford these drugs.
- Doses as high as 15 mg/kg twice daily may be needed to control clinical signs.
- Treatment success is monitored with an ACTH stimulation test, which should be performed 1-3 hours after ketoconazole administration.
- Potential side effects include anorexia, vomiting, lightening of the hair coat, increase in liver enzymes, and hepatotoxicity.
-
- Adrenal-dependent hyperadrenocorticism (ADH):
- Surgery:
- Surgery is curative for adrenal adenomas or small carcinomas. Prognosis is usually good if the patient survives the first 2 weeks post-surgery.
- However, adrenalectomy may be associated with a high rate of intra- and post-operative complications including death. It should not be performed prior to abdominal ultrasound evaluation of tumor location, size, invasion of vessels, and organs. The surgery should be performed by a qualified and experienced surgeon.
- Complications include cardiac arrest, pulmonary thromboembolism (check for risks such as high systemic blood pressure, decreased anti-thrombin III levels), acute renal failure, pneumonia, pancreatitis, and acute adrenal insufficiency.
- If hyperkalemia and hyponatremia occur, oral fludrocortisone (0.01 to 0.02 mg/kg daily) is administered.
- During and after surgery, glucocorticoid must be administered to prevent rapid development of adrenocortical insufficiency (the contralateral adrenal is atrophied).
- Dexamethasone (0.05 to 0.1 mg/kg) IV is administered immediately before surgery and immediately after surgery for a period of about 6 hours. After that, administer it subcutaneously twice at the day of surgery and then, two to three times a day until the dog starts eating and does not have vomiting and/or diarrhea (parental glucocorticoids should not be given longer than 3 days).
- ACTH stimulation test is performed 24 to 48 hours after surgery. Perform it before administering the daily glucocorticoid dose.
- Prednisone/solone at the dose of 0.25 to 0.5 mg/kg (lower dose to larger dogs and higher dose to smaller dogs) twice daily should be given as soon as possible after surgery. Reduce the dose gradually and adjust the dose if the animal shows signs of hypoadrenocorticism during the process. The minimal amount of steroid should be given until the remaining gland has regained function, as determined by the ACTH stimulation test. Typically, in 3 to 6 months the dogs can be completely off of prednisone/solone.
- Surgery:
- Adrenal-dependent hyperadrenocorticism (ADH):
Important Facts
- Surgery is curative for adrenal adenomas or small carcinomas.
- Adrenalectomy may be associated with a high rate of intra- and post-operative complications including death; therefore, careful consideration should be exercised to determine if the patient is a good candidate for surgery.
- If hyperkalemia and hyponatremia occur, oral fludrocortisone is administered.
- An ACTH stimulation test should be performed 2 to 3 days after surgery to evaluate adrenal reserve and exclude occult metastasis or incomplete resection.
- Glucocorticoid has to be administered before surgery, during surgery, and after surgery until the remaining gland has regained function.
-
-
- Trilostane:
- It can also be used to treat adrenal tumors with the same protocol described above for PDH.
- Lysodren® (o.p-DDD):
- Indications for Lysodren® therapy in cases of ADH are:
- Gross metastatic disease evident before surgery.
- Unresectable or incompletely resectable tumor.
- Residual disease after adrenalectomy.
- Unacceptable anesthetic and surgical risks.
- Refusal of surgery by the owner.
- The protocol used to manage PDH will be applied for treatment of ADH.
- The induction dose is higher (50 to 100 mg/kg) and the induction period is on average longer (10 to 14 days).
- The maintenance dosage is on average higher.
- Indications for Lysodren® therapy in cases of ADH are:
- Trilostane:
-
Important Facts
- The same Lysodren® protocol used to manage PDH can be used to manage ADH.
- On average, the induction and maintenance dosages will be higher and the induction period longer.
-
-
- Ketoconazole:
- At this time, the primary use for ketoconazole in dogs with ADH is for preoperative preparation. It is administered during 4 to 8 weeks before adrenalectomy.
- Ketoconazole:
-
References
Arenas C, Melian C, Perez-Alenza MD. Evaluation of 2 trilostane protocols for the treatment of canine pituitary-dependent hyperadrenocorticism: twice daily versus once daily. J Vet Intern Med 2013; 27:1478-1485.
Behrend EN, Kooistra HS, Nelson R et al. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (Small Animal). J Vet Intern Med 2013; 1292-1304.
Bennaim M, Shiel RE, Mooney CT. Diagnosis of spontaneous hyperadrenocorticism in dogs. Part 1: Pathophysiology, aetiology, clinical and clinicopathological features. Vet J 2019; 252: 105342. doi: 10.1016/j.tvjl.2019.105342
Bennaim M, Shiel RE, Mooney CT. Diagnosis of spontaneous hyperadrenocorticism in dogs. Part 2: Adrenal function testing and differentiating tests. Vet J 2019; 252: 105343. doi: 10.1016/j.tvjl.2019.105343
Bugbee A, Rucinsky R, Cazabon S et al. 2023 AAHA selected endocrinopathies of dogs and cats guidelines. J Am Anim Hosp Assoc 2023; 59:113-135.
Feldman E.C. & Nelson R.W. Canine Hyperadrenocorticism (Cushing’s Syndrome). In: Canine and Feline Endocrinology and Reproduction. 3rd ed. Philadelphia: W.B. Saunders, 2004; 252-357.
Guptill L, Scott-Moncrieff JC, Widmer WR. Diagnosis of canine hyperadrenocorticism. Vet Clin North Am Small Anim Pract 1997; 27(2): 215-235.
Kintzer PP & Peterson ME. Diagnosis and management of canine cortisol-secreting adrenal tumors. Vet Clin North Am Small Anim Pract; 1997; 27(2):299-307.
Lathan P. Laboratory diagnosis of thyroid and adrenal disease. Vet Clin Small Anim 2023; 53:207-224.
Mack RE, Feldman EC, Wilson SM. Diagnosis of hyperadrenocorticism in dogs. Compend Contin Educ Pract Vet 1994; 16(3): 311-347.
Ramsey, IK. Trilostane in dogs. Vet Clin Small Anim 2010; 40:269-83.
Reid LE, Behrend EN, Martin LG. Effect of trilostane and mitotane on aldosterone secretory reserve in dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med 2014; 28:443-450.
Rodriguez Pineiro MI, Benchekroun G, de Fornel-Thibaud P et all. Accuracy of an Adrenocorticotropic hormone (ACTH) immunoluminometric assay for differentiating ACTH-dependent from ACTH-independent hyperadrenocorticism in dogs. J Vet Intern Med 2009; 23:850-855.

