1.9 Canine Flank Alopecia
-
General Considerations
- Other names for this condition include seasonal flank alopecia, cyclic flank alopecia, or recurrent flank alopecia. Because it is not always seasonal, cyclic, or recurrent the best terminology is simply “flank alopecia”.
- The pathophysiology of flank alopecia is unknown. However, there are many evidences supporting the role of light. In addition, breed predisposition suggests that genetic also plays a role.
- Anecdotally, Vizslas, German wirehaired pointers, and the Cesky Fousek breed can develop an atypical form of recurrent flank alopecia. In this form, the alopecia is more severe and, in addition to the flanks, it may affect the sacral area, thighs, base of the tail, and in some cases the ears, nose, and rarely the head. Eventually, the alopecia may progress and become persistent. In contrast to the typical form, the alopecic skin is not hyperpigmented.
- In the northern and southern hemispheres, the clinical signs develop during the months with shorter duration of light, and typically resolve during the spring and summer months where the daylight duration is much longer.
- Anecdotal reports indicate that exposing affected dogs to sun lamps stimulate hair regrowth.
- The exact influence of light duration on hair growth in such localized body area is not known but one can speculate that the pineal gland stimulates the secretion of certain hormones that will act in specific hair follicle receptors that are increased in density or hyper-responsiveness in the dogs that develop this condition.
-
Clinical Signs
- Most dogs develop the disease between 3 to 6 years of age (mean 3.8 years).
- Boxers, French bulldogs, Airedale terriers, and English bulldogs are at higher risk but the condition has been also described in all sizes of schnauzers, miniature poodles, Doberman pinchers, Bouvier de Flanders, Chesapeake Bay retriever, Scottish terriers, Staffordshire terrier, German shorthair pointer, and other breeds.
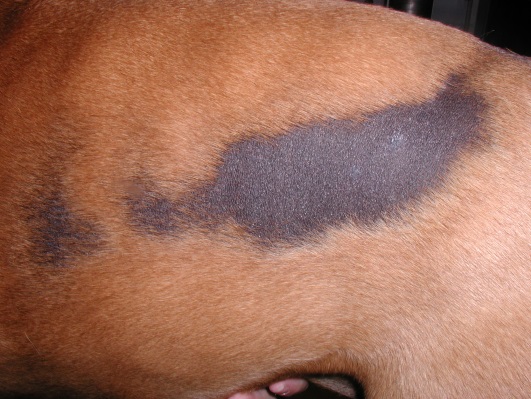
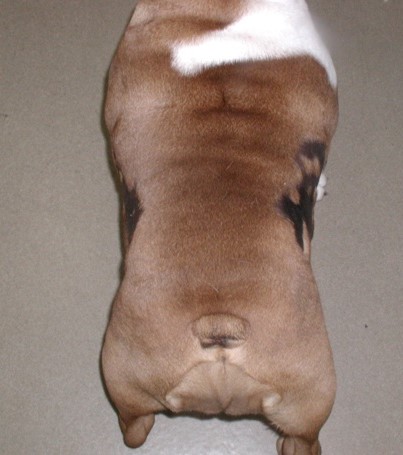
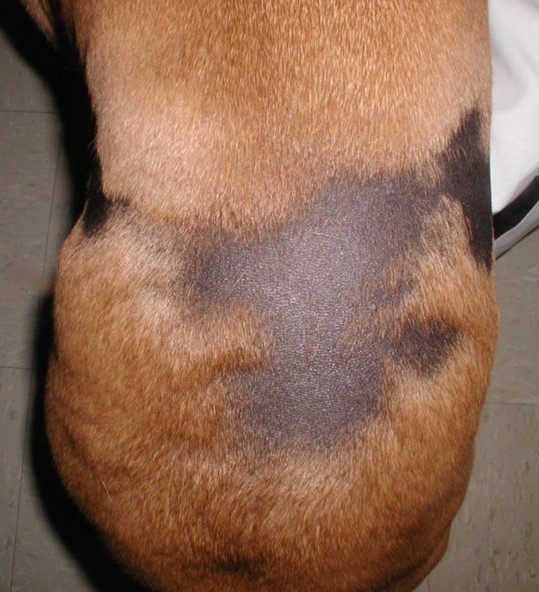
- Bilateral non-inflammatory alopecia more often localized to the dorsolateral thoracic-lumbar region. Lesions can occasionally occur on the dorsum and cross the dorsal midline. They are usually annular to polycyclic in shape and have well-demarcated borders. The adjacent skin and hair are typically normal in contrast with most endocrine disorders.
-
- The alopecic skin is usually markedly hyperpigmented.
-
- Scales, when present, are fine and small.
- In some dogs, only one side of the body is affected or one side is more severely affected than the other side.
- Although not always, most cases will have a cyclic and recurrent pattern. Most dogs in the northern hemisphere will lose hair between November and April and regrow it spontaneously in the spring or summer; about 20% of the dogs are reported to only have one episode of hair loss. The cycling and alopecia episodes may vary in dogs from southern locations.
- Dogs may not develop alopecia in some years or the condition is milder and of shorter duration.
- It may take from 1 to 14 months for the hair to regrow.
- Regrown hair may be of different color and it is typically darker than previous hairs.
- In some cases, the alopecia becomes persistent (seen more often in Airedales).
- Secondary bacterial and Malassezia infection or overgrowth may develop and induce pruritus.
-
Diagnosis
- The differential diagnoses include hypothyroidism, hyperadrenocorticism, Alopecia X and sex hormone-related alopecias.
- Definitive diagnosis should be based on a characteristic history, typical clinical signs, and laboratory test results that rule out other conditions.
- Skin biopsy shows dysplastic hair follicles, which have an octopus or jellyfish like appearance. Sebaceous glands can be melanized. These findings are not specific and can be found in any endocrine diseases that affect the skin.
-
Treatment
- There is currently no effective therapy for flank alopecia and because affected dogs are healthy otherwise, benign neglect is a reasonable option.
- Melatonin implants, injections, or oral melatonin has been used to manage this condition with variable results and should be considered an option if owners request treatment. Three melatonin implants of 12 mg (total dose 36 mg) and two 12.5 mg subcutaneous injections at 2-week interval were given to Airedales, boxers, and giant Schnauzers in one report. The recommended dosage of oral melatonin is 3 mg (small dogs) to 6 mg (large dogs) per dog every 8 to 12 hours.
- Some dogs continue to develop recurrent seasonal hair loss for years; other dogs have an occasional year when the alopecia does not occur; still other dogs eventually develop permanent alopecia.
Important Facts
- The pathophysiology of flank alopecia is unknown.
- Bilateral alopecia and hyperpigmentation more often localized to the thoracic and lumbar regions are typical clinical signs. The lesions are usually annular to polycyclic in shape and have well-demarcated borders.
- Most dogs in the northern hemisphere lose hair in the late fall and regrow it spontaneously in the spring or summer; 20% of cases only experience one episode of alopecia.
- The alopecia may become permanent in some dogs.
- Airedale terriers, English bulldogs, and boxers are at higher risk but the condition has been described in many other breeds.
- Diagnosis should be based on a characteristic history, typical clinical signs, and laboratory test results that rule out other conditions.
- Skin biopsy shows dysplastic hair follicles, which have an octopus or jellyfish like appearance. Sebaceous glands can be melanized.
- There is currently no effective therapy for flank alopecia but melatonin is a safe and inexpensive option when owners request therapy.
References
Albanese F, Malerba E, Abramo F et al. Deslorelin for the treatment of hair cycle arrest in intact male dogs. Vet Dermatol 2014; 25:519-288.
Cerundolo R, Lloyd D.H., Persechino A et al. Treatment of canine Alopecia X with trilostane. Vet Dermatol 2004; 15: 285-293.
Cerundolo R, Warren S. The use of deslorelin to promote hair regrowth in dogs with Alopecia X, in Proceedings. 26th Annual Congress of the ESVD-ECVD 2013; 184.
Cerundolo R, Mauldin EA, Goldschmidt MH et l. Adult-onset hair loss in Chesapeake Bay retrievers: a clinical and histopathological study. Vet Dermatol 2005; 16: 39-46.
DeClementi C, Bailey K.L., Goldstein SC et al. Suspected toxicosis after topical administration of minoxidil in 2 cats. J Vet Emerg and Crit Care 2004; 14(4):287-292.
Frank L.A. Hnilica K.A., Oliver JW. Adrenal steroid hormone concentrations in dogs with hair cycle arrest (Alopecia X) before and during treatment with melatonin and mitotane. Vet Dermatol 2004; 15:278-284.
LaFort-Dassot C, Beco L, Carlotti NE. Follicular dysplasia in five Weimaraners. Vet Derm 2002; 13:253-260.
Layne EA and Richmond RV. Deslorelin implant treatment for hair cycle arrest (Alopecia X) in two intact male keeshonden. J Am Hosp Assoc 2018; 54. DOI 10.5326/JAAHA-MS-6646.
Lopes CEB, Lundberg A, White A, et al. Pathology in practice: Alopecia in a boxer. J Am Vet Med Assoc 2024; doi.org/10.2460/javma.24.04.0286.
Lothrop, CD Jr.: Pathophysiology of growth hormone responsive dermatosis. Compend. Cont. Ed. 1998; 1346-1352.
Miller WH, Griffin GE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis: Elsevier Inc., 2013; 537-540.
Miller WH, Griffin GE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis: Elsevier Inc., 2013; 554-572.
Miller WH, Griffin GE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis: Elsevier Inc., 2013; 593-599.
Miller WH Jr, Scott DW. Follicular dysplasia of the Portuguese water dog. Vet Dermatol 1995; 6:67-74.
Miller MA, Dunstan RW. Seasonal flank alopecia in boxers and Airedale terriers: 24 cases (1885-1992). J Am Vet Med Assoc 1993; 203:1567-1572.
Muntener T, Schuepbach-Regula G, Frank L, et al. Canine noninflammatory alopecia: a comprehensive evaluation of common and distinguishing histological characteristics. Vet Dermatol 2012; 23: 206-e44. DOI: 10.1111/j.1365-3164.2012.01049.x.
Neradilova S, Schauer AM, Hayward JJ, et al. Genomic and transcriptomic characterization of atypical
recurrent flank alopecia in the Cesky Fousek. Genes 2022; https://doi.org/10.3390/genes13040650.
Parker HG, Chase K, Cadieu E, et al. An insertion in the RSPO2 gene correlates with improper coat in the Portuguese water dog. J Hered 2010; 10:612-617.Parker WM and Scott DW. Growth hormone responsive alopecia in the mature dog: A discussion of 13 cases. J Am Anim Hosp Assoc 1980; 16(6): 824-828.
Schmeitzel LP & Lothrop CD Jr. Hormone abnormalities in Pomeranians with normal coat and those with growth hormone responsive dermatosis. J Am Vet Med Assoc 1990; 15: 1333-1341.
Schmeitzel LP. Sex hormone-related and growth hormone-related alopecias. Vet. Clin. North. Am. (Small Anim Pract) 1990; 20: 1579-1601.
Scott DW. Seasonal flank alopecia in ovariohysterectomized dogs. Cornell Vet 1990; 80:187-195.
Stoll S. et al. Microneedling as a successful treatment for alopecia X in two Pomeranian siblings. Vet Dermatol 2015.
Vandenabeele S, Declercq J, De Cock H, et al. Canine recurrent flank alopecia: a synthesis of theory and practice. Vlaams Diergeneeskundig Tijdschrift 2014; 83.
Welle M, Philippe U, Rüfenacht S, et al. MLPH genotype—melanin phenotype correlation in dilute dogs. J Hered 2009;100: S75–S79.

