4.11 Entomophthoromycosis and Mucormycosis (both previously known as Zygomycosis) – Small and Large Animals
Learning Objectives
- Know that causative agents of entomophthoromycosis and mucormycosis penetrate the body via the respiratory, gastrointestinal tracts or wound contamination.
- Know these fungal diseases can manifest in two forms: subcutaneous or visceral. The subcutaneous form is characterized by nodules, which can ulcerate and drain. The visceral form is manifested as abdominal mass, vomiting, diarrhea and oral ulcerations. The visceral form has a poor prognosis.
- Describe the clinical presentation of basidiobolomycosis and conidiobolomycosis in horses.
- Know that the diagnosis is based on a detailed history and characteristic clinical signs in addition to cytology, biopsy and culture results. The cytological exam of aspirates, direct smears of exudate from draining lesions and histopathology reveal a pyogranulomatous inflammation rich in eosinophils with the presence of wide sparsely septate hyphae. Fungal organisms grow in Sabouraud’s dextrose agar and the preferred sample is a skin tissue.
- Know how to manage this disease.
- Remember! The prognosis is guarded for the subcutaneous form and it is poor for the visceral form of the disease.
-
General Considerations
- Fungal species of entomophthoromycetes, basidiobolomycetes and mucoromycetes are ubiquitous saprophytes of soil, air, water and decaying vegetation and are a component of the normal flora of the skin and hair coat. They are the causative agents of entomophthoromycosis and mucormycosis, previously called zygomycosis.
- The agents of entomophthoromycosis belong to the phylum Zoopagomycota, which were previously placed under the phylum Zygomycota. The agents of mucormycosis belong to the phylum Mucoromycota.
- The portal of entry of the fungal organisms may be gastrointestinal, respiratory, or cutaneous via wound contamination.
- In humans, an immunocompromised host is necessary for infection to establish. However, in dogs, cats, and horses such factors have not been identified in most cases.
- In the U.S., entomophthoromycosis and mucoromycosis are most commonly seen in states along the Gulf of Mexico. Entomophthoromycosis caused by species of Basidiobolus and Conidiobolus have been also reported in Africa, Asia, Australia and South America, which are tropical and subtropical regions. Mucoromycete organisms are ubiquitous but also more often seen in subtropical and tropical climates.
- Agents of entomophthoromycosis and mucoromycosis are nonpigmented fungal organisms (i.e. hyalo hyphae).
Important Facts
- Fungal species of entomophthoromycetes, basidiobolomycetes and mucoromycetes are ubiquitous saprophytes of soil, water and decaying vegetation and are a component of the normal flora of the skin and hair coat.
- These fungi are the causative agents of entomophthoromycosis and mucoromycosis, previously known as zygomycosis.
- The fungal organisms penetrate the body via respiratory and gastrointestinal tracts or wound contamination.
-
Animal Species Affected
- Entomophthoromycosis and/or mucoromycosis have been reported in humans, dogs, cats, sheep, llama and horses.
-
Causative Agents
- Mucoromycetes:
- Rhizopus spp., Rhizomucor spp., Mucor spp., Absidia spp., Saksenaea spp., Mortierella spp, Cokeromyces spp.
- Entomophthoromycetes:
- Conidiobolus spp.
- Basidiobolomycetes:
- Basidiobolus spp.
- Mucoromycetes:
Important Facts
-
- Entomophthoromycosis and/or mucoromycosis have been reported in humans, dogs, cats, sheep, llama and horses.
-
Clinical Signs
- Dogs and Cats:
- Subcutaneous form:
- Solitary or multiple nodules, which are typically ulcerated and draining.
- It commonly involves the extremities.
- Regional lymphadenopathy may occur.
- Conidiobolus organisms typically cause nodular lesions on the external nares and inside the nasal passages leading to nasal discharge, facial or nasal deformity, ulceration of the nasal planum or hard palate, and exophthalmos with possible extension to the skin or the brain.
- Basidiobolus organisms have been reported to cause ulcerative cutaneous lesions in dogs resembling pythiosis.
- Visceral form:
- The visceral form is characterized by the presence of one or more of the following: abdominal mass, vomiting, diarrhea and oral ulcers.
- Basidiobolus spp. infection can cause gastrointestinal, respiratory and disseminated disease.
- The visceral form can be fatal.
- Subcutaneous form:
- Horses:
- Mucormycosis:
- Mucormycosis appears to be rare in horses. The disease caused by Absidia corymbifera has been reported in two horses.
- Clinical signs are characterized by single or multiple, ulcerative and granulomatous lesions localized to the limbs and face.
- Basidiobolomycosis:
- Caused by Basiodiobolus haptosporus.
- No age, sex or breed predilection exist and lesions occur throughout the year.
- Large (up to 60 cm in diameter), circular, ulcerative lesions with a serosanguineous discharge are most commonly found on the chest, trunk, head, and neck. The pictures below are good examples for such lesions.
- Mucormycosis:
- Dogs and Cats:
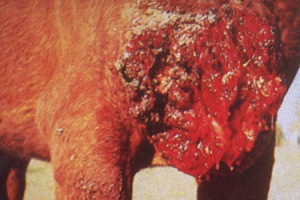
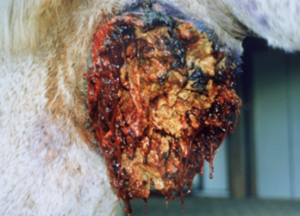
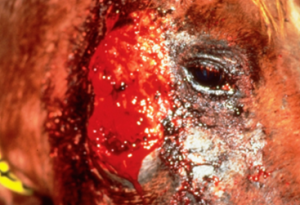
-
-
-
- Solitary lesions are the rule and pruritus is usually present.
- The leeches that characterize pythiosis are not always present and, if present are smaller (less than 0.5 mm) than the ones seen with pythiosis.
-
-
-
-
- Conidiobolomycosis:
- Caused by Conidiobolus coronatus.
- There is no age, sex or breed predilection.
- Lesions are found on the external nares or in the nasal passage or both. They can be single or multiple, unilateral or bilateral.
- Lesions in the nasal passages are firm nodules covered by an edematous, focally ulcerated mucosa. Lesions of the external nares appear grossly similar to those of basidiobolomycosis. Hemorrhagic nasal discharge and dyspnea may be present due to nasal blockage.
- The leeches that characterize pythiosis are often present but are smaller (less than 0.5 mm).
- Conidiobolomycosis:
-
-
Diagnosis
- History and clinical signs.
- Cytology: Cytological exam of aspirates or direct smears from exudative lesions reveals granulomatous to pyogranulomatous inflammation rich in eosinophils in the case of entomophthoromycosis. The fungal organisms are similar to oomycetes and characterized by wide (Conidiobolus 5-15 µm; Basiodiobolus ~ 30 µm) and sparsely septate hyphae with nonparallel walls and haphazard branching. The inflammatory reaction associated with mucormycosis is characterized by a pyogranulomatous inflammation. The features of the fungal organisms vary according to the causative agent. Rhizomucor, Rhizopus, and Saksenaea spp. have large, variably sized (5-20 µm), sparsely septate, irregular hyphae. Mucor spp. show cigar-shaped to round yeast-like cells and hyphae. To facilitate the visualization of the organisms, Grocotti methenamine silver (GMS) and periodic acid shiff (PAS) stains should be used. A wet-mount can also be prepared to visualize the organisms: 10 to 20% KOH plus India ink.
- Incision biopsy: Histopathology shows a nodular to diffuse, superficial and deep granulomatous to pyogranulomatous to necroulcerative inflammation. Eosinophils may be present in large numbers in the case of entomophthoromycosis and amorphous material forming an eosinophilic sleeve around the hyphae can be seen. The presence of wide, thin-walled, irregularly branching with non-parallel sides and occasionally septate hyphae will support the diagnosis of entomophthoromycosis and mucormycosis. Fungal elements are most commonly found within foci of necrosis and are best visualized with Grocotti methenamine silver stain (GMS). They stain variably with period acid Schiff (PAS) and do not stain with hematoxylin and eosin (H&E) showing hyphae ghosts or light blue staining of hyphae with H&E.
- Fungal culture: The organisms grow in Sabouraud’s dextrose agar. Incision biopsy tissues and/or vigorously washed leeches are the preferred material for culture. Tissue should not be ground or macerated because this will destroy the organisms.
- Molecular tests: PCR tests provide a rapid identification of the causative agents.
- Differential diagnoses:
- Dogs: other subcutaneous or deep fungal or bacterial infection, mycobacterial infection, foreign body, sterile panniculitis, sterile pyogranuloma, neoplasia.
- Horses: other fungal and bacterial granulomas, exuberant granulation tissue, habronemiasis, fibroblastic sarcoid, squamous cell carcinoma, pythiosis.
Important Facts
- Cytology of aspirates, direct smears of exudate and histopathology reveal a pyogranulomatous inflammation rich in eosinophils with the presence of wide, thin-walled and occasionally septate hyphae.
- The organisms grow in Sabouraud’s dextrose agar.
- Incisional biopsies and/or leeches are the preferred material for culture.
- PCR tests have the advantage of providing a rapid identification of the causative agents.
-
Treatment
- Solitary lesions may be surgically excised.
- Aggressive surgical excision when feasible or debulking should be followed by antifungal chemotherapy.
- Oral potassium or sodium iodide (0.5 to 1.2g /30kg q 24h), amphotericin B, miconazole, itraconazole (10 mg/kg q 24h) or newer triazoles (posaconazole, voriconazole) are options to consider.
- Ideally, antifungals should be selected based on susceptibility testing but these tests are not readily available.
- Months of therapy are typically needed, minimum of 2 to 3 months.
- Treatment instituted early in the disease process has better prognosis.
Important Facts
- Aggressive surgical excision when feasible or debulking should be followed by antifungal chemotherapy.
- Oral potassium or sodium iodide, amphotericin B, miconazole, itraconazole are antifungal options; however, selection should be based on culture and susceptibility testing.
- A minimum of 2-3 months of therapy is generally required.
-
Prognosis
- Dogs:
- Subcutaneous form is guarded.
- Visceral form is poor.
- Horses:
- Fair to good if diagnosed early.
- Dogs:
References
Barrs VR, Bęczkowski PM, Talbot JT et al. Invasive fungal infections and oomycoses in cats. 1. Diagnostic approach. J Fel Med Surg 2024; doI: 10.1177/1098612X231219696
Barrs VR, Hobi S, Wong A et al. Invasive fungal infections and oomycoses in cats. 2. Antifungal therapy. J Fel Med Surg 2024; doI: 10.1177/1098612X231220047
Dehghanpir SD. Cytomorphology of deep mycoses in dogs and cats. Vet Clin Small Anim 2023; 53: 155–173.
Greene CE. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, Missouri, Elsevier, Saunders, 2012.
Guillot J, Collobert C, Jensen HE, et al. Two cases of equine mucormycosis caused by Absidia corymbifera. Equine Vet J 2000; 32: 453–456.
Hillier A, Kunkle GA, Ginn PE et al. Canine subcutaneous zygomycosis caused by Conidiobolus sp.: A case report and review of Conidiobolus infections in other species. Vet Dermatol 1994; 5: 205-213.
Hoffmann AR, Ramos MG, Walker RT et al. Hyphae, pseudohyphae, yeasts, spherules, spores, and more: A review on the morphology and pathology of fungal and oomycete infections in the skin of domestic animals. Vet Pathol 2023; doi.org/10.1177/03009858231173715
Miller WH, Griffin CE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis, Missouri, Elsevier, Mosby, 2013.
Prabhu R and Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect 2004; 10: 31-47.
Rippon JW. Medical Mycology. Philadelphia, WB Saunders, 1988.
Scott DW. Large Animal Dermatology. Philadelphia, WB Saunders, 1988.
Skiada A and Petrikkos G. Cutaneous zygomycosis. Clin Microbiol Infect 2009; Suppl 5: 41-45.
Spatafora JW, Cang Y, Benny GL, et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016; 108: 1028–1046.

