4.10 Phaeohyphomycosis – Small Animals
Learning Objectives
- Know that phaeohyphomycosis is caused by saprophytic fungi found in the soil and organic material, and that the infection occurs via wound contamination.
- Know that the fungal elements are characteristically pigmented and have broad septate, branched or unbranched hyphae.
- Remember that clinically the disease resembles mycetoma (subcutaneous nodules with ulcer and draining tracts) but no grains are present on exudate from draining tracts.
- Know that the diagnosis is based on a detailed history, characteristic clinical signs, and cytological, histopathological and fungal culture findings.
- Know that PCR can be used for rapid identification of the fungal species.
- Know which samples should be collected for cytological exam and fungal culture.
- Know the cytology and histopathology findings of an animal with phaeohyphomycosis.
- Learn how to manage phaeohyphomycosis.
-
General Considerations
- Subcutaneous, cutaneous, cerebral or systemic infection caused by dematiaceous (dark pigmented) septate fungi.
- The fungi are ubiquitous saprohytic organisms found in various soils and organic materials.
- Infection occurs via wound contamination.
- Phaeohyphomycosis differs from mycetoma in that the involved fungi do not organize to form grains.
- Immunosuppressive therapy may increase the risk of infection; however, many patients are not taking immunosuppressive drugs by the time of diagnosis.
Important Facts
- Dark pigmented fungi cause the disease.
- The fungi are saprophytic organisms present in soil and organic materials and penetrate the body via wound contamination.
- In contrast to mycetomas, no grains are present in the exudate from draining tracts.
-
Animal Species Affected
- The disease has been reported in dogs, cats, and horses.
-
Causative Agents
- Examples of saprophytic agents of phaeohyphomycosis include Drechslera spp., Bipolaris spp., Moniliella spp., Exophiala spp., Phialophora spp., Cladophialophora spp., Fonsecaea spp., Stemphyllium spp., Lecytophora spp., Pseudomicrodochium spp., Phialemonium spp., Alternaria spp., Hormodendrum spp, and Curvularia spp..
-
Clinical Signs
- Dogs:
- Solitary or multiple dermal and subcutaneous poorly defined and often ulcerated nodules. These nodules may evolve to develop draining tracts.
- Dogs:
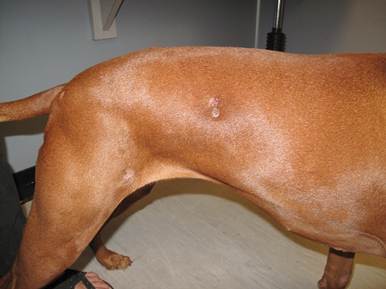
-
-
- Lesions are more often present on the nose and distal extremities, which are sites frequently in contact with the contaminated soil.
- Lesions may be grossly pigmented and can be mistaken for melanoma.
- Contiguous skeletal and disseminated infections are uncommon but occur more often in dogs than in cats. Systemic infection with multifocal skin lesions, involvement of lymph nodes and distant organs develop more frequently in immunosuppressed animals.
- Cats:
- Slow growing, firm to fluctuant, cutaneous to subcutaneous nodules, which can become ulcerated and form draining tracts.
-
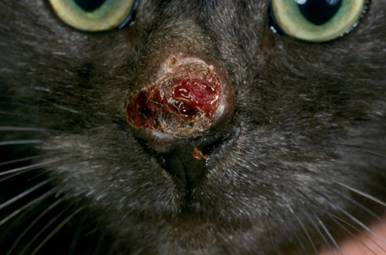
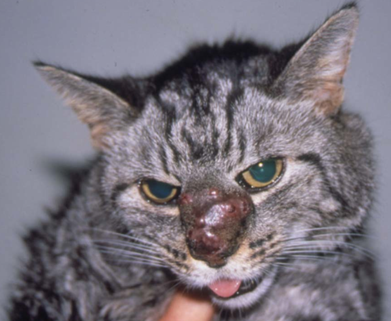
-
-
- In most cases, lesions are solitary and affect the foot, leg, ears, nose and trunk.
- Lesions may be grossly pigmented and can be mistaken for melanoma.
- Disseminated infection is rare and occurs more often in immunocompromised animals.
-
Important Facts
- Single or multiple cutaneous to subcutaneous nodules that may ulcerate and form draining tracts.
- Disseminated infection is rare and occurs more often in immunocompromised animals.
-
Diagnosis
- Cytology – Aspirates from nodules or direct smears of exudate from draining lesions reveal a pyogranulomatous inflammation. The presence of pigmented fungal elements supports the diagnosis. A Romanowsky stain will show various intensities of the fungal melanin as blue-green or green-brown. However, some fungal organisms may stain basophilic with no obvious melanin. Fontana-Mason staining may highlight the melanin in lightly pigmented fungal cells. However, not all pigmented fungi have a positive reaction and some agents of hyalohyphomycosis can produce some melanin when grown in culture. For more detail on the cytological features of the fungal organisms, refer to the paper by Dehghanpir 2023.
- Skin Biopsy – Pyogranulomatous inflammation associated with numerous fungal elements characterized by broad pigmented and septate hyphae. Fontana-Masson stain and Grocott methenamine silver stain (GMS) highlight the melanin in the fungal cell wall and should be requested if the H&E stain is negative. For histopathologic description of the fungal organisms, refer to the paper by Hoffmann et al, 2023.
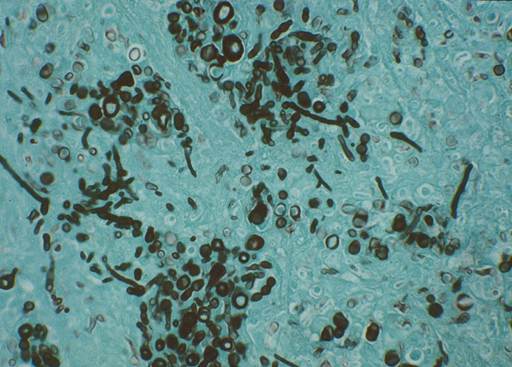
-
- Fungal Culture – Fungal organisms grow in Sabouraud’s dextrose agar at 25°C to 35°C. Biopsy tissue is the preferred sample for culture.
- PCR may be used for rapid identification of the causative agent.
Important Facts
- Cytological exam of aspirates from nodules, direct smears of exudate from draining lesions and skin biopsy reveal a pyogranulomatous inflammation with the presence of pigmented and septate fungal elements.
- Fungal organisms grow in Sabouraud’s dextrose agar.
- Biopsy tissues are the preferred sample for culture.
- PCR can be used for rapid identification of the causative fungus.
-
Treatment: For antifungal dose protocols not included below, refer to the Blastomycosis chapter
- Wide surgical excision of solitary lesions may be curative but recurrences are common. Unfortunately, depending on the lesion location surgical resection with wide margins may not be feasible.
- Antifungal chemotherapy should be prescribed for several months after lesion resection.
- Medical therapy should be continued for 12 months or more in animals with nonresectable disease. Some of these cases still fail treatment or the disease relapses after discontinuation of the antifungal.
- Antifungal chemotherapy should be based on in vitro susceptibility testing.
- The response to the various drugs is unpredictable.
- The response to the various antifungals is unpredictable.
- Itraconazole alone or in combination with 5-flurocytosine has been successfully used. Itraconazole dose: Dogs – Loading dose of 20 mg/kg then 10 mg/kg q 24h. Cats – 10 mg/kg q 24h.
- Amphotericin B alone or in combination with 5-flurocytosine can be tried for severe cases that do not respond to itraconazole. Amphotericin B dose: Dogs – 1-3 mg/kg IV 3 times weekly to a cumulative dose of 12-27 mg/kg. Cats: 1 mg/kg IV 3 times weekly to a cumulative dose of 12 mg/kg.
- Posaconazole and voriconazole may be more effective than itraconazole but are more expensive. Voriconazole is the drug of choice for intracranial phaeohyphomycosis in dogs because it reaches high concentrations in the central nervous system. Remember to use voriconazole carefully in cats because of the risk for serious side effects. Posaconazole dose: Dogs – 5 mg/kg q 48h. Cats – Loading dose of 15 mg/kg followed by 7.5 mg/kg q 24h. Alternative protocol – Loading dose of 30 mg/kg, followed by 15 mg/kg q 48h. Voriconazole dose: Dogs – 4-5 mg/kg q 12hr.
- Terbinafine has been shown to potentiate the effect of azoles and should be considered in cases that are not responding to azoles as sole therapy. It has been successful as monotherapy in some cases in people.
- Local application of heat to potentiate the effect of antifungals has been used in humans.
Important Facts
- Wide excision can sometimes be curative for solitary lesions.
- Antifungal chemotherapy should be used after surgical resection of the lesion and drug selection should be based on in vitro susceptibility testing.
- Months of antifungal therapy is required in most cases.
References
Arcobello JT and Revankar SG. Phaeohyphomycosis. Semin Respir Crit Care Med 2020; 41: 131-140.
Barrs VR, Bęczkowski PM, Talbot JT et al. Invasive fungal infections and oomycoses in cats. 1. Diagnostic approach. J Fel Med Surg 2024; doI: 10.1177/1098612X231219696
Barrs VR, Hobi S, Wong A et al. Invasive fungal infections and oomycoses in cats. 2. Antifungal therapy. J Fel Med Surg 2024; doI: 10.1177/1098612X231220047
Dedeaux A, Grooters A, Wakamatsu-Utsuki N et al. Opportinistic fungal infections in small animals. J Am Anim Hosp Assoc 2018; DOI 10.5326/JAAHA-MS-6768
Dehghanpir SD. Cytomorphology of deep mycoses in dogs and cats. Vet Clin Small Anim 2023; 53: 155–173.
Greene CE. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, Missouri, Elsevier, Saunders, 2012.
Hoffmann AR, Ramos MG, Walker RT et al. Hyphae, pseudohyphae, yeasts, spherules, spores, and more: A review on the morphology and pathology of fungal and oomycete infections in the skin of domestic animals. Vet Pathol 2023; doi.org/10.1177/03009858231173715
Miller WH, Griffin CE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis, Missouri, Elsevier, Mosby, 2013.
Rippon JW. Medical Mycology. Philadelphia, WB Saunders, 1988.
Scott DW. Large Animal Dermatology. Philadelphia, WB Saunders, 1988.
Swift IM, Griffin A, Shipstone MA. Successful treatment of disseminated cutaneous phaeohyphomycosis in a dog. Aust Vet J 2006; 84: 431-435.

