4.1 Dermatophytosis – Dogs and Cats
Learning Objectives
- Know that dermatophytosis is overdiagnosed.
- Know that clinical signs are quite variable. Therefore, a direct examination of hairs/scales and/or fungal culture is necessary to confirm a presumptive diagnosis.
- Remember that most cases that look like ringworm in dogs are caused by staphylococcal infection.
- Remember that wood’s lamp may give false negative and false positive results.
- Be able to describe the technique used to collect hairs and scales for direct examination and fungal culture.
- Learn when the Mackenzie toothbrush technique is applicable.
- Know that DTM, Sabouraud’s or RSM media can be used for dermatophyte isolate. Keep the media plate at room temperature and check it daily for change in color (if using DTM or RSM) and for colony growth.
- Learn how soon change in media color and fungal growth can be noted when infected samples are cultured.
- Learn how long you should wait before declaring a culture result negative.
- Learn the characteristics (color and texture) of dermatophyte colonies of the three fungal organisms causing dermatophytosis in dogs and cats.
- Learn the characteristics (color and texture) of the colonies associated with saprophytic fungi.
- Can saprophytic organisms change the media color as dermatophytic organisms do? If yes, could you explain the mechanism?
- Know that you should recommend treating the environment when managing a case of dermatophytosis (spores can survive in the environment for years and can be a source of infection).
- Learn the recommendations to best treat the environment (include products and other measures you think are helpful). You will need to educate the client.
- Remember that topical therapy should not be used as the sole treatment to manage feline dermatophytosis.
- Learn the best dip solutions to manage dermatophytosis in cats and dogs.
- Know the options for systemic therapy.
- Learn the potential side effects associated with griseofulvin in cats and how to monitor for side effects.
- Remember! Do not stop therapy before obtaining at least one (ideally two) negative fungal culture. Treatment duration typically lasts 7 to 8 weeks.
-
General Considerations
- Dermatophytes invade keratinized structures of the skin and its adnexa, e.g. hair, horn, nails, feathers, and stratum corneum.
- Dermatophytosis is a self-limiting condition:
- However, it may take several months or longer to spontaneously resolve.
- The host immune status influences the severity and course of the disease.
- It is a zoonotic disease; therefore, treatment is generally instituted.
Important Facts
- Dermatophytosis is a self-limiting condition but it may take months to resolve spontaneously.
- It is a zoonotic disease, so treatment is typically instituted.
-
Cause
- Most dermatophytes that infect dogs and cats are zoophilic. However, these organisms can also infect humans.
- Zoophilic agents of dermatophytosis in dogs and cats include Microsporum canis, Microsporum persicolor and Trichophyton mentagrophytes.
- Geophilic dermatophytes inhabit the soil and decompose keratinaceous organic debris. The only geophilic species known to cause disease in dogs and cats is Microsporum gypseum.
- Antropophilic dermatophytes primarily infect humans but can rarely infect animals.
- Most cases of dermatophytosis in dogs and cats is caused by Microsporum canis. However, Trichophyton mentagrophytes, Microsporum gypseum and Microsporum persicolor can also cause disease.
- Hair parasitism:
- Endothrix – fungal arthrospores are produced inside the hair shaft. These are anthropophilic organisms.
- Ectothrix – fungal arthrospores are produced outside the hair shaft. These are zoophilic and geophilic dermatophytes. Therefore, the large majority of dermatophytosis cases in animals will be associated with an ectothrix parasitism.
Important Facts
- Microsporum canis is the most common cause of dermatophytosis in cats and dogs. However, Trichophyton mentagrophytes, Microsporum gypseum and Microsporum persicolor can also be causative agents.
- Most cases of dermatophytosis in animals will be associated with an ectothrix parasitism.
-
Clinical prevalence of dermatophytosis
- Dermatophytosis is an overdiagnosed condition, especially in dogs.
- The infection is most common in very young and old animals, in immunosuppressed animals or when animals are housed in conditions of poor sanitation and overcrowding (e.g. “puppy mills”, pet shops, and catteries).
- Dermatophytosis appears to occur more frequently in warm and humid tropical areas.
- Breed predilection is difficult to determine because dermatophytosis is not a reportable disease, it can resolve spontaneously and it is not fatal.
- Yorkies appear to be more prone to generalized and dermal/subcutaneous infections typically caused by Microsporum canis.
- There is evidence that Persian cats are more prone to dermatophytosis, typically caused by Microsporum canis. Moreover, they are overrepresented in all reports of dermal/subcutaneous infections (i.e. mycetoma/pseudomycetoma).
- Hunting and working dog breeds appear to be predisposed to Microsporum persicolor and Microsporum gypseum infections.
Important Facts
- Dermatophytosis is overdiagnosed, especially in dogs.
- Very young and old or immunosuppressed animals are predisposed to infection.
- Dermatophytosis appears to occur more frequently in warm and humid tropical areas.
-
Transmission
- The most common mode of infection caused by Microsporum canis is by direct contact with an infected host, typically a cat. However, transmission can also occur via fomite such as, grooming appliances, collars, bedding, and exposure to a contaminated environment.
- Despite the fact that fungal spores can remain viable in the environment for months, transmission from contaminated environments is generally not very effective. Infections caused by Microsporum gypseum (geophilic) are presumed to occur by direct contact with contaminated soil. Most Trichophyton spp. infections are suspected to result from contact with rodents or their nests.
- Animal to human (most common) and human to animal transmissions can occur.
- Asymptomatic carriers (especially cats) can serve as reservoir of infection.
- Ectoparasites (e.g. fleas, Cheyletiella) especially in catteries and multiple cat households may serve as mechanical vectors and cause skin microtrauma, which is needed for the infection to establish.
Important Facts
- The main mode of transmission is direct contact with an infected host. However, fomites and contaminated environments also play a role.
- Remember! Fungal spores can remain viable in the environment for months.
- Remember! Asymptomatic carries (especially cats) can serve as reservoir of infection.
-
Pathophysiology
- The dermatophyte must invade keratin of the stratum corneum and/or hair. In experimental studies, skin microtrauma has shown to be a pre-requisite for infection to establish.
- The organisms only grow in anagen (growing) hairs.
- Growing hairs contain carbohydrates, nitrogenous substances, and nucleoprotein derivatives in addition to keratin.
- The fungi grow downward to just above the hair bulb.
- The hair shaft is weakened and breaks.
- Infection induces the hair follicle to enter telogen (resting stage). Then the fungal organisms stop growing in that hair. However, by that time it has spread to a neighboring hair.
- Dermatophytes can prevent host response to infection by (i) inhibiting lymphocytes by cell wall mannas, (ii) altering macrophage function, and (iii) slowing keratinocyte turnover.
- Clinical signs associated with dermatophytosis are reflections of the host’s response to the fungus.
- Well-adapted species (e.g. Microsporum canis) will typically induce minimal inflammation.
- Less well-adapted species (e.g. Microsporum gypseum, Trichophyton mentagrophytes) will typically induce more marked inflammation.
- Microsporum canis infection can also induce severe inflammatory lesions if hair follicles rupture (furunculosis) exposing the fungal elements and hair shaft to the dermis. These inflamed and draining lesions are called kerion. As mentioned previously, Yorkies are more prone to develop deep infections.
- Inflammation expels the fungus from the infected hair shaft and the infection spreads to peripheral hairs.
- Incubation periods vary from 4 to 40 days.
Important Facts
- Well-adapted species (e.g. Microsporum canis) will typically induce minimal inflammation.
- Less well-adapted species (e.g. Microsporum gypseum) will induce marked inflammation, which will cause more discomfort to the animal but may help reduce the duration of the infection.
- Inflammation expels the fungus from the hair shaft but the infection progresses to peripheral hairs.
- Incubation periods vary from 4 to 40 days.
-
Immunology
- Cell-mediated immunity is most important.
- No correlation has been found between circulating dermatophyte-specific antibodies and protection.
Important Facts
- Cell-mediated immunity is more important for the animal’s protection against infection than an antibody response.
-
Clinical Signs
- Canine Dermatophytosis:
- Variable clinical signs:
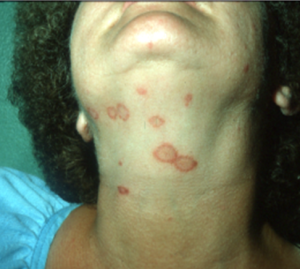
- The “classic” lesion is a circular patch of alopecia characterized by broken stubby hairs, scaling, and mild erythema. Lesion appears to spread outward and often leave a central healing.
- Variable clinical signs:
- Canine Dermatophytosis:

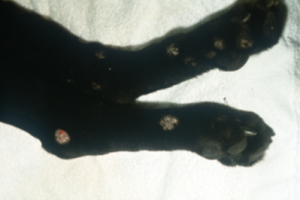

-
-
-
- Dermatophyte lesions can be focal, multifocal, or generalized. In most cases, it is limited to keratinized structures (i.e. hair shaft, stratum corneum, nails) but occasionally it can occur in the dermis (i.e. kerion-see below) and subcutaneous tissue (mycetoma/pseudomycetoma).
- Generalized conditions warrant search for an underlying cause.
- Papules and pustules can be present in some cases. The pustular form may mimic pemphigus foliaceus.
- Pruritus is usually NOT present in dogs.
- Major differential diagnoses include superficial pyoderma and demodicosis. Remember, dermatophytosis is usually overdiagnosed in dogs!
-
-
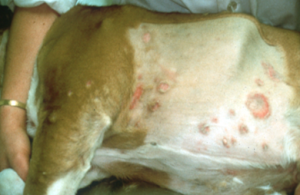
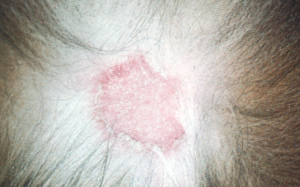
Important Facts
- Clinical signs are quite variable.
- Most cases that look like ringworm in dogs will be caused by staphylococcal pyoderma. Epidermal collarette lesions mimic the classic ringworm lesion (i.e. patch of alopecia characterized by broken stubby hairs, scaling, and mild erythema).
-
-
- Other Clinical Presentations:
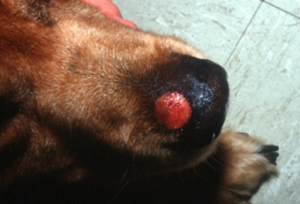
- Kerion:
- Nodular, dermal inflammatory reaction with draining tracts that results from rupture of hair follicles and exposure of fungal elements and hair shafts to the dermis (these act as foreign material). Some lesions may ulcerate.
- Kerion:
- Other Clinical Presentations:
-
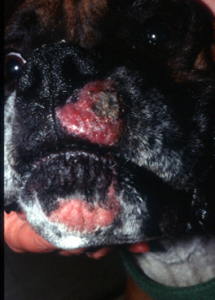
-
-
-
-
- Differential diagnoses for a kerion lesion include deep pyoderma, neoplasia (histiocytoma, mast cells tumor, etc.), and localized demodicosis with furunculosis.
-
-
-
Important Facts
- Kerion is a nodular lesion associated with draining tracts resulting from the rupture of infected hair follicles and the severe inflammatory reaction induced by the presence of the fungus elements and hair shafts in the dermis.
-
-
-
- Onychomycosis:
- Dermatophyte infection of the nail. It is rare.
- Onychomycosis causes abnormal nail growth and brittle nails (onychodystrophy). Paronychia (inflammation of the nail fold) can also be present. It is usually asymmetric and, it rarely occurs by itself.
- It is most often caused by Trichophyton mentagrophytes.
- Onychomycosis can be difficult to diagnosis.
- It usually requires prolonged systemic therapy.
- Onychomycosis:
-
-
Important Facts
- Onychomycosis is a rare condition caused by dermatophyte infection of the nail. The infection can extend to the nail fold.
- It can be difficult to diagnose and to manage.
- Usually, other parts of the body are also involved.
-
- Feline Dermatophytosis:
- Microsporum canis is the most common cause of dermatophytosis in cats.
- Dermatophytosis can become endemic in some catteries, especially in longhaired cats.
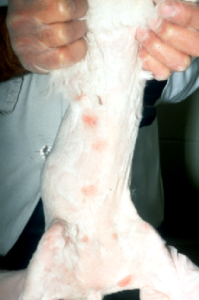
- Clinical Signs:
- Patches of alopecia, crusting, scaling, especially localized to the face, ears and paws but lesions can be present anywhere in the body.
- Feline Dermatophytosis:

-
-
-
- Miliary dermatitis (i.e. multiple, small papules covered with crusts) may be present.
- Some cats can be asymptomatic carriers – in infected catteries, only the kittens may be clinically affected.
- Pruritus is common and can be intense in some cats.
- Pseudomycetoma:
- Pseudomycetoma is caused by dermatophyte infection of the dermis and subcutaneous tissue.
- Lesions are characterized by subcutaneous nodules with draining tracts typically with grains in the exudate.
- It is most commonly seen in Persian cats.
- Pseudomycetoma is typically very difficult to manage.
- Check the immune status of cats with the generalized form (FeLV, FIV).
-
-
Important Facts
- Dermatophytosis in cats is typically associated with alopecia, crusting and scaling localized to the face, ears and paws.
- Pruritus is common in cats and can be severe in some cases.
- Remember! Cats can be asymptomatic carriers.
- Pseudomycetoma is characterized by dermal to subcutaneous nodules with draining tracts with or without grains mixed in the exudate. It is most commonly seen in Persian cats.
-
Diagnosis
- No single diagnostic test should be considered “gold standard”. Various tests should be used in combination such as (i) Wood’s lamp and direct examination of hairs to document active infection, (ii) dermatophyte culture to identify the fungal species and monitor response to therapy and (iii) biopsy for nodular or atypical clinical signs.
- Wood’s Lamp Examination:
- Wood’s lamp has an UV light of 253.7 nm that is filtered through a cobalt or nickel oxide filter.
- The stability of the light wavelength and the intensity of the light are temperature dependent. It may requires a few minutes to warm up.
- Lamps with built-in magnification lens facilitate examination and interpretation of results.
- The fluorescence is caused by a water-soluble chemical metabolite known as pteridine that results from the infection of hair shafts (fluorescence is not present in scales or crusts). It has been noted to develop as soon as 5-7 days post-infection but, more often 10-14 days.
- The only species of veterinary importance that fluoresce is Microsporum canis. Fluorescence will be seen in about 70% of the cases if the user has experience using the lamp and has an adequate instrument. Sensitivity will likely reduce if the animal has been treated.
- Color seen: apple or blue green on hair shaft. Be sure that the hair root (i.e. portion present inside the follicle) also fluoresces.
- Crusts covering infected hairs will obscure the fluorescence so, make sure to lift any crusts and examine the underneath hairs.
- False positive results can occur. Medications adhered to hairs, scales and crusts can falsely fluoresce. Remember, only hairs fluoresce! The false positive fluorescence is not apple or blue green.
- Keep in mind that negative results do not rule out dermatophytosis.
- The Wood’s lamp is useful to select suspected hairs for direct examination and fungal culture.
- Do not use the Wood’s lamp to monitor response to therapy because the fluorescence may last for a long time after the infection is resolved.
- A positive Wood’s lamp should not be considered a definitive diagnosis.
- Wood’s lamp has an UV light of 253.7 nm that is filtered through a cobalt or nickel oxide filter.

Important Facts
- About 70% of Microsporum canis strains fluoresce under the Wood’s lamp. Therefore, negative results do not rule out dermatophytosis.
- Only the infected hair shaft fluoresces with the Wood’s lamp. If scales and crusts fluoresce, the fluorescence should be considered false.
- The Wood’s lamp is helpful to select suspected hairs for direct examination and fungal culture. A positive result should not be considered a definitive diagnosis.
- Do not use the Wood’s lamp to monitor response to therapy because the fluorescence may last for a long time after the infection is resolved.
-
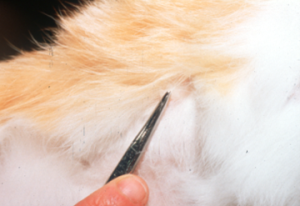
- Direct Examination of Hairs (also known as trichogram) and Scales:
- Pluck hairs (with roots) with a small hemostat. Be sure to select broken hairs from within and close to the periphery of the lesion. Do not sample the nice hairs bordering the lesions. Plucked hairs are better samples than scales.
- Direct Examination of Hairs (also known as trichogram) and Scales:
-
-
-
- Wood’s lamp can help select hairs.
-
-
-
-
- After plucking hairs, apply mineral oil to a scalpel blade and scrape the skin superficially in the affected area to collect scales. Make sure to pluck affected hairs first because scraping may cut hair shafts making it difficult to pluck them.
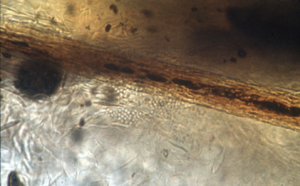
- Place the sample on a few drops of mineral oil, place a cover slip on top and examine first under low power to look for broken and abnormal hairs, then at 40X for better visualization. Lower the microscope condenser to increase contrast, as fungal elements (i.e. arthrospores and hyphae) are transparent.
- Spores form outside of hair shafts (i.e. ectothrix parasitism) – they are translucent balls and are not pigmented. They are in chains or aggregates.
-

-
-
- Hyphae can be found in association with fungal arthrospores but they can be difficult to see.
- Do not confuse with cotton fiber, thread, fiberglass, etc.
- Remember, a negative direct examination does not rule out dermatophytosis.
- Hyphae can be found in association with fungal arthrospores but they can be difficult to see.
-
Important Facts
- A negative direct examination of hairs and scales does not rule out dermatophytosis!
- Accurate sample collection increases your the chances to find the organisms.
- Pluck the abnormal hairs (broken) located within the lesion. Do not sample the nice hairs bordering the lesion.
- Samples can be visualized using 10% KOH or simply mineral oil.
- Look for spores and/or hyphae parasitizing the outside of the hair shaft (ectothrix parasitism).
-
- Fungal Culture: It is used not only to identify the dermatophyte species but also to monitor response to therapy.
- Specimen Collection:
- Hair: most commonly cultured specimen.
- Obtain broken hairs from within active lesions.
- Gently pluck hairs in the direction of growth to remove the root.
- Scales:
- Scrape superficially using a sterile scalpel to collect scales within active lesions. Remember to pluck hairs before performing skin scraping because the act of scraping will cut hair shafts and you may not be able to pluck them.
- Nails:
- Scrape the most ventral portion of the nail plate that appears abnormal.
- Look for fragments of affected nails that can be easily removed.
- A more successful, but more traumatic technique is to avulse the nail or clip a large portion of the nail as close to the nail bed as possible.
- Kerion and pseudomycetoma.
- Tissue culture – biopsy the skin using a 2mm punch instrument and submit the sterile sample for culture. Hairs on the surface of the kerion or pseudomycetoma nodules may not be parasitized; therefore, the tissue culture is important to confirm the clinical diagnosis.
- Histopathology is also important to confirm the presumptive clinical diagnosis. Place the biopsy sample immediately in 10% formalin and submit to the laboratory with a detailed patient’s history, test results if available and, ideally, photos of the lesions.
- In some cases the exudate from draining tracts may contain fungal elements so, perform cytology of the draining exudate.
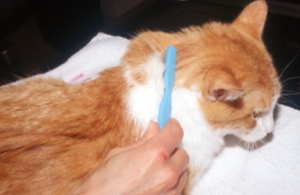
- Mackenzie toothbrush technique: It is used more frequently to collect samples from asymptomatic carries; however, it can also be used to sample lesions.
- Brush the animal’s hair coat vigorously using a sterile soft bristle toothbrush.
- Brush multiple areas of the body, specially the face, pinnae and paws which are often affected until the bristles are full of hair .
- Hair: most commonly cultured specimen.
- Specimen Collection:
- Fungal Culture: It is used not only to identify the dermatophyte species but also to monitor response to therapy.

-
-
- Deposit the sample firmly (do not imbed) in the culture media. When using the toothbrush technique to collect samples, make sure to stab the bristles onto the surface of the medium in four to five different areas. Moreover, touch the right and left sides of the bristles on the medium surface.
- If no previous medications, fungal growth may occur in 5 to 7 days.
- However, it may take 3 weeks if the animal is on therapy. Therefore, wait 3 weeks before declaring a fungal culture negative.
- Color change of the media may occur in 4 to 7 days.
- Keep the medium at room temperature and examine it daily! In the presence of dermatophyte organisms, the change in color of the DTM medium occurs before or simultaneously with the colony growth and it typically occurs within the first week. In the case of contaminants, the medium color will occur after the colony growth and typically after 10 to 14 days.
- Culture Media:
- Sabouraud’s dextrose agar: used for all fungal cultures.
- Rapid sporulation medium (RSM): It encourages formation of macroconidia, which is used to identify the fungus. Glucose and bromothynol blue are indicators. Dermatophytes turn the medium blue-green early.
- Dermatophyte Test Medium (DTM) – It contains the following ingredients:
- Sabouraud’s dextrose agar.
- Cyclohexamide – inhibits saprophytic fungal growth.
- Gentamicin, chlortetracycline – inhibits bacteria growth.
- Phenol red – pH indicator.
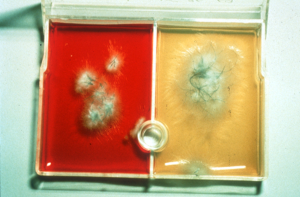
- Dermatophytes utilize protein first producing ammonia and an alkaline pH. Therefore, the media turns red at the first evidence of colony growth (usually the colony is visible at about 7 days).
- If dermatophytes stay in the culture plates too long, they deplete the protein in the medium and begin utilizing the carbohydrates. This will cause the medium to turn back to yellow.
- Dermatophyte colonies are white (Microsporum canis) or light tan (Microsporum gypseum) in color and fluffy (Microsporum canis) or granular (Microsporum gypseum) in texture.
- Contaminant (saprophytic) fungi utilize carbohydrate first. Therefore, the media will stay yellow at the first sign of colony growth.
- It is important to check the media daily to know when the color will change, which will help determine if the fungus is pathogenic or saprophytic (contaminant).
- Some species of Alternaria, Aspergillus and Penicillium will turn the media red after the colony has been growing for a short period. Usually the colonies of saprophytic fungi are green to black (i.e. pigmented).
- Microscopic evaluation of the colony is important to make the diagnosis.
-
-
-
-
- Stained Slide Preparation:
- Examine all suspect colonies growing on DTM, Sabouraud’s or RSM medium.
- Pick up a sample from the surface of the colony with a clear acetate tape. Place the tape on a glass slide flooded with lactophenol cotton blue. Add more lactophenol cotton blue if needed and cover with a coverslip.
- Examine the sample for the presence of macroconidia.
- Stained Slide Preparation:
-
-
-
- PCR test:
- PCR test is available for the diagnosis of dermatophytosis.
- False positive and negative results can occur.
- Do not use PCR test to monitor response to therapy because non-viable fungal fragments can be amplified resulting in a false positive result and unnecessary longer treatment duration.
- The same samples (i.e. hair, scales or crusts) used for direct examination and culture are submitted for PCR.
- The turnaround time is 2 to 3 days and IDEXX currently provide this service.
- PCR test:
Important Facts
- Use the same technique previously described to collect samples for direct examination to collect samples for fungal culture.
- The Mackenzie toothbrush technique is used more frequently to collect hair samples from asymptomatic cats. However, it can also be used to collect samples from lesions.
- DTM, Sabouraud’s or RSM medium can be used for dermatophyte culture.
- Keep the medium at room temperature and examine it daily!
- In the presence of dermatophyte organisms the change in color of the medium, when using DTM, occurs before or simultaneously with the colony growth.
- In the case of contaminants, the medium-color change will occur after the colony growth and typically after 10 to 14 days.
- It may take up to 3 weeks before any growth can be seen. Therefore, wait at least 3 weeks before giving a negative result.
- Dermatophyte colonies are white or light tan in color; saprophyte colonies are usually pigmented (e.g. green, black).
- Examine all colonies under the microscope for the characteristic features of the fungal macroconidia.
- PCR test is available to diagnose dermatophytosis.
- Do not use PCR test to monitor response to therapy because non-viable fungal fragments can be amplified resulting in a false positive result and unnecessary longer treatment duration.
- The turnaround time for PCR tests is 2 to 3 days.
-
Treatment
- Reasons to treat:
- Decrease the risk for disease spreading to other animals.
- Reduce the chances for environmental contamination.
- Hasten recovery from infection.
- Dermatophytosis is a zoonotic disease.
- Topical:
- Single Lesions:
- Spot treatment with topical antifungal creams or lotions.
- Clip affected area a few inches beyond the edge of the lesion.
- Miconazole, clotrimazole, cuprimyxin, haloprogin, cyclopirox, povidone-iodine, or thiabendazole.
- Apply two times daily. Prevent the animal from licking or rubbing the treated areas for at least 30 minutes after application.
- Whole body treatment with topical dip is helpful to eliminate fungi from clinically non-affected areas (lime-sulfur, enilconazole – not available as dip solution in the US).
- Avoid treating cats with topicals as the sole therapy! Remember, cats can be carriers of dermatophytes and topical therapy may not eliminate the carrier state.
- Topical therapy can be used as the only therapy for focal and mild cases in dogs.
- Spot treatment with topical antifungal creams or lotions.
- Multiple Lesions:
- Clip affected areas a few inches beyond the edge of the lesion.
- Consider clipping the whole body in severe generalized cases. Advantages include (i) it may help reduce environmental contamination by removing infected hairs and (ii) clipping allows easier skin contact of topical products. Disadvantages include (i) infection may spread mechanically causing initial worsening of clinical signs, and (ii) will need to sedate the cat for whole body clipping. Be sure to dispose off the hair carefully if performing whole body clipping.
- Treat the entire body twice (ideally) to once weekly.
- Lime sulfur – diluted 4 oz. /gallon. Cats should wear an E-collar until the dip solution is dry to avoid significant ingestion and potential side effects. Interestingly, a study reported that none of the cats treated bi-weekly with lime sulfur dips (up to 15 treatments) wore a device during treatment and none showed any side effect.
- Enilconazole (20 mls/liter) – labeled as environmental product in the USA – Clinafarm®EC.
- Dilute to 0.2% (14.5 ml of the 13.8% concentrate added to 1L of water – ref.2).
- Apply about 100 ml to the cat’s dry hair coat twice weekly.
- Potential side effects associated with topical enilconazole (Clinafarm®EC) are salivation, idiopathic muscle weakness, increase in ALT, anorexia, weight loss and vomiting. However, these adverse reactions are usually transient and benign. Cats should wear an E-collar until the solution is dry to avoid significant ingestion and potential side effects.
- 2% chlorhexidine + 2% miconazole shampoo has been shown to reduce the duration of systemic therapy and performed better than shampoos with either ingredient. Bathe two tothree times per week.
- Avoid using topical therapy solely to manage feline dermatophytosis because the medication may not penetrate effectively the hair follicle canal and treat the portion of the hair shaft inside the canal.
- Single Lesions:
- Reasons to treat:
Important Facts
- Avoid spot treating cats as the sole therapy! Remember, cats can be healthy carriers.
- Clip the affected and surrounding areas every time you are using topical therapy.
- Whole body dip is a good adjuvant therapy for cases associated with multiple lesions.
- Lime sulfur and enilconazole have been shown to have better effect than the other topical products.
- Shampoos containing a combination of chlorhexidine and miconazole have been shown to be effective as adjunctive therapy.
-
- Systemic:
- Itraconazole (do not compound):
- 5 to 10 mg/kg once daily. Administer with food to improve absorption.
- A labeled protocol for cats is as follows: 7 days of itraconazole at 5 mg/kg orally q 24h followed by 7 days off itraconazole. This treatment protocol can reduce cost and potential itraconazole side effects.
- Itraconazole therapy provides more rapid cure than ketoconazole in cats.
- It has fewer sides effects than ketoconazole. Side effects include one or more of the following:
- Elevated liver enzymes.
- Vomiting and/or diarrhea
- Cutaneous vasculitis in dogs. This side effect is typically seen if given at doses higher than 10 mg/kg/day.
- Terbinafine (Lamisil®):
- 30-40 mg/kg q 24h dose has been efficacious.
- It has been efficacious to treat pseudomycetomas as sole therapy or in conjunction with surgery to reduce nodule size before excision.
- Increase in liver enzymes has been reported but no clinical signs of liver disease may be seen. Monitor liver enzymes during treatment.
- Vomiting and diarrhea may also occur but are rare side effects.
- Griseofulvin:
- It is fungistatic – effective only against dermatophytes.
- Adverse reactions:
- It is teratogenic.
- With the exception of vomiting, nausea and diarrhea are rare. Divide the dose and give with food to reduce the chances for side effects.
- Elevated liver enzymes can occur during therapy and it can be hepatotoxic.
- Cats may be very sensitive to griseofulvin:
- Panleukopenia due to bone marrow suppression may be associated with FIV positive cats or it may be idiosyncratic. Therefore, perform a CBC every 7 to 14 days in every cat receiving griseofulvin.
- Anorexia, vomiting, diarrhea, and lethargy are most commonly seen.
- Other side effects include angioedema, ataxia, depression and fever.
- Side effects may be more common in Persian, Himalayan, Siamese, and Abyssinian breeds.
- Dose:
- Fulvicin U/F (microsized): 50 to 150 mg/kg q 24h divided in twice or three times daily administrations. The authors usually use 50 mg/kg q 24h.
- Gris-PEG, Fulvicin P/G (ultramicrosized, polyethylene glycol base): 5 to 10 mg/kg q 24h divided in twice daily administrations.
- Pediatric suspension is available.
- Give with a fatty food to improve absorption.
- Do not use in animals less than 6 weeks of age.
- Ketoconazole:
- Not as effective as itraconazole, terbinafine and griseofulvin. Avoid it in cats.
- Recommended dose is 10 mg/kg q 24h.
- Give with an acidic or acid-producing meal (e.g. canned food, tomato juice).
- Hepatitis, vomiting and/or diarrhea have been reported. Cats are more sensitive to the side effects of ketoconazole than dogs; therefore, only use ketoconazole in cats if you do not have another option.
- Fluconazole:
- Not as effective to treat dermatophytosis as itraconazole, terbinafine, griseofulvin and ketoconazole.
- Recommended dose is 5-10 mg/kg q 24h. Food is not required for optimal absorption.
- Generally well tolerated. However, vomiting, diarrhea and dose related increase in serum ALT may be seen during treatment.
- Lufenuron:
- Considering the controversial and discouraging results of various studies, at this time lufenuron should not be used to treat dermatophytosis.
- Length of Therapy:
- Independent of the treatment regimen chosen for each patient, treat until at least one negative culture is obtained. Ideally, treat until two negative fungal cultures are obtained at 2 weeks interval.
- Usually a minimum of 8 weeks of therapy is required. Therefore, it is important to know what you are treating. Do not perform a treatment trial!.
- Itraconazole (do not compound):
- Systemic:
Important Facts
- Systemic therapy should always be considered in the management of feline dermatophytosis.
- Itraconazole, terbinafine and griseofulvin are most effective.
- Remember! Cats can develop bone marrow suppression with griseofulvin. FIV cats are more susceptible. Perform a CBC every 7 to 14 days.
- Cats are very sensitive to the side effects of ketoconazole; avoid this drug in cats.
- Itraconazole and terbenifine are considered safe in cats and are more efficacious than ketoconazole.
- Fluconazole is the least effective antifungal for dermatophytosis.
- Lufenuron has no convincing effect against dermatophytes and should not be used.
- Be sure to treat until at least one (ideally two) negative culture is obtained!
- Usually, a minimum of 8 weeks is necessary to obtain cure but this will vary among cases.
-
- Environment decontamination: The main purpose to treat the environment is to prevent fomite contamination (mostly cats) and false positive fungal culture results.
- Make sure to clean and dry the surfaces before using disinfectants.
- Disinfect areas with dilute bleach (1 part of bleach to 10 parts of water) at least two times weekly.
- Enilconazole (Clinafarm®EC) is an effective environmental disinfectant at the concentration of 20 microliters/L – at least two times weekly.
- Accelerated hydrogen peroxide is available in concentrates, in ready to use formulations and over the counter products. Its efficacy against Microsporum canis and Trichophyton spp. has been shown in various studies. Disinfect twice weekly.
- Over the counter general disinfectants with label indicating fungicidal action against Trichophyton mentagrophytes have shown to be effective when applied liberally and with a 10 minute contact time.
- One study tested various commercial disinfectants in vitro against Microsporum canis spores and found the following to be effective if 1 to 5 spray volumes are applied to a 16 cm2 area: quaternary ammonium 0.3% (409®; The Clorox Company); sodium hypochloride 1.84% (Clorox Clean-UP®; The Clorox Company), lactic acid 3.2% (Lysol®, Reckitt Benckiser), and hydrogen peroxide 0.5% (Accel® TB; Virox Technologies).
- Change heating and air conditioning filters and clean vents.
- Environment decontamination: The main purpose to treat the environment is to prevent fomite contamination (mostly cats) and false positive fungal culture results.
Important Facts
- Always recommend cleaning and disinfecting the environment!
- Under ideal environmental conditions, fungal spores can survive in the environment for months!
- The environment can be disinfected with bleach (1:10 dilution), enilconazole, accelerated hydrogen peroxide and general disinfectants with labels indicating fungicidal action against Trichophyton mentagrophytes.
- Change heating and air conditioning filters and clean vents.
-
- Multiple Cat Households/Catteries:
- Perform toothbrush fungal cultures (i.e. Mackenzie toothbrush technique) in all cats in a cattery and all animals in the household (i.e. clinically and non-clinically affected animals).
- Dip all cats in the household or cattery/shelter with lime sulfur when waiting for fungal culture results.
- Ideally, culture-positive asymptomatic cats should be treated as culture positive symptomatic cats, i.e. add systemic therapy to the topical.
- Segregate infected from uninfected cats:
- Reculture negative cats as they may become positive when recultured.
- Another option is to assume that all cats are culture positive and treat them as positive.
- Treat until all animals are culture negative at least once. Ideally twice at two-week interval.
- Clean and disenfect the environment!
- Preventive measures:
- Isolate and culture all new cats.
- Pending culture results, dip (lime sulfur) all new cats weekly.
- Culture and dip cats returning from cat shows.
- Remember: dermatophytosis is a zoonosis.
- Multiple Cat Households/Catteries:
References
Kunder D and Moriello K. Efficacy of eight commercial disinfectants against Microsporum canis spores on textile swatches. Vet Dermatol 2012; 23: 85.
Hnilica and Medleau. Evaluation of topically applied enilconazole for the treatment of dermatophytosis in a Persian cattery. Vet Dermatol 2002; 13:23.
Moriello K, Coyner K, Paterson S, Mignon B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2017; 28:266-e68.
Scott, Miller, Griffin. Small Animal Dermatology. In: Fungal Skin Diseases. W. B. Saunders, Philadelphia, PA, 1995, p 332 – 350.

